
Annual Report
2022
Annual Report
2022

I
Chair’s letter
Medical progress and innovation are evolving with impres-
sive speed even in aworld of increasing volatility and
uncertainty. In 2022, we initiated a major transformation
of our organization to further improve our innovation capa-
bilities and align with our growth strategy as a pure-play
medicines company.
We expect these changes to simplify our business,
enhance accountabilities and strengthen our commer-
cial activities by enabling us to better focus on our in-mar-
ket products and high-value pipeline assets. Overall, our
eorts are set to support our long-term sales and profit
growth and help us create sustainable shareholder value.
As part of this transformation, which includes our inten-
tion to spin o our Sandoz generics and biosimilars Divi-
sion, we merged our Oncology and Pharmaceuticals com-
mercial organizations and created a new Operations
organization that combines our manufacturing and ser-
vices activities. Besides sub stantial cost savings, we
expect these steps to increase our operational agility and
strengthen our business in key markets such as the
United States and China.
The organizational changes, which we expect to finalize
in 2023, complete the portfolio shift we started in 2014.
Over this time, we have divested several non-core busi-
nesses and spun o our eye-care division Alcon. With a
view to boosting our innovation power, we have also made
substantial investments in cutting-edge technology plat-
forms, including gene andcell therapy, radioligand ther-
apy and RNAtechnology.
Novartis delivered on its sales and operating profit tar-
gets in 2022 despite the challenges of a volatile macro-
economic environment and while executing on our ongo-
ing transformation, which entailed jobreductions due to
structural changes. This performance was supported by
cost discipline and continued operational streamlining,
as well as the strong uptake of recently launched
medicines, such as multiple sclerosis treatment Kes i
-
mpta, and continued strong demand for our cardiovas-
cular medicine Entresto and psoriasis treatment
Cosentyx.
Last year also saw new leadership at the Novartis Insti-
tutes for BioMedical Research and our Global Drug
Development organization, the research and develop
-
ment (R&D) engines of Novartis, and a renewed focus on
improving governance and speeding up the transition
between early drug discovery and clinical development.
These measures should help further strengthen our port-
folio of medicines, which we have consistently broadened
in recent years with launches including cancer treat-
ments Pluvicto andScemblix.
We have also further intensified our eorts to integrate
our environmental, social and governance (ESG) activi-
ties into our daily business, which we believe is vital for
the success of Novartis. Among a variety of steps aimed
at reaching as many patients as possible, we renewed
our commitment to continue our R&D eorts in neglected
tropical diseases and to work further toward the elimi-
nation of malaria. In addition, we are partnering with the
American Society of Hematology to deepen our eorts
to fight against sickle cell disease in Africa.
The Board of Directors also remained vigilant in its gov-
ernance oversight eorts by putting added emphasis on
values such as integrity as Novartis pivots towards
becoming a high-performance, pure-play medicines
company. Likewise, the Executive Committee and the
Board of Directors are keeping up the intensive dialogue
with stake holder groups and working towards achieving
our vision to be the most valued and trusted medicines
companies in the world.
I thank you for the confidence you have placed in our
company and am pleased to be able to propose a divi-
dend increase of 3.2% to CHF 3.20 at the next Annual
General Meeting.
Sincerely,
Joerg Reinhardt
Chair of the Board of Directors

II
CEO’s letter
2022 was a year of transformation for Novartis. After more
than USD 100 billion in acquisitions and divestures over
the last several years, our structural transformation from
a diversified healthcare conglomerate into a focused,
innovative medicines company will be largely complete
after the planned spin-o of Sandoz in 2023.
We also begin 2023 with a simplified organizational struc-
ture that will spur innovation and give us a stronger foun-
dation for growth in a rapidly changing global business
environment.
While Novartis has pursued bold portfolio change, core
elements of our company remain the same. Our vision is
to become the most trusted and valued medicines com-
pany in the world – valued not only for our business per-
formance, but also for the dierence our innovation makes
for patients and society.
The world is counting on us to succeed. Fewer than 10%
of diseases known to aect humans are currently treat-
able, and globally, people live an average of 10 years with
a disease or disability. Yet new treatments broadly still
reach only a fraction of eligible patients, and manageable
conditions like heart disease cause millions of avoidable
deaths each year.
Our performance in 2022 showed that we are making
progress in addressing society’s greatest disease bur-
dens. Our focus on cardiovascular disease, for example,
gives countries and healthcare systems solutions to
address the world’s leading cause of death and disability.
Entresto, our medicine for heart failure and hypertension,
is estimated to be treating around 10 million patients
worldwide, while our cholesterol-lowering siRNA treat-
ment Leqvio is now approved in 70 countries.
We saw robust growth momentum across our in-market
medicines. This includes stronger-than-expected uptake
in the US for Pluvicto, our novel radioligand therapy for
advanced prostate cancer, highlighting our ability to turn
the promise of next-generation medicines into a reality for
patients.
Despite challenging macroeconomic conditions and an
unstable geopolitical environment, we delivered a solid
financial performance that underscores the progress we
are making, with 4% growth in net sales in constant cur-
rencies (cc) and 8% growth (cc) in core operating income
compared with the previous year. Looking ahead, we aim
to generate sales growth of 4% CAGR over the next five
years, and grow above peer median beyond 2027.
Our investments in R&D are key to achieving these ambi-
tions. In 2022, we saw a positive Phase III readout for ipta
-
copan, which was discovered at NIBR, in a rare and deadly
blood disorder. We saw an important positive Phase III
result for Pluvicto in earlier lines of prostate cancer. And
we also reported positive Phase III results for Cosentyx in
hidradenitis suppurativa, oering the potential to expand
one of our most successful medicines and bring a new
treatment option to patients with this painful skin disease.
As we continue innovating for patients, millions around the
world are still without proper access to healthcare. Trans-
lating the latest science into lasting progress requires us
to work with healthcare systems and other stakeholders to
advance access for underserved patients in low- and mid-
dle-income countries, while also tackling access barriers
in some of the wealthiest countries in the world.
In the US, for example, we expanded our 10-year Beacon
of Hope initiative, which seeks to address racial dispari-
ties in healthcare, including by increasing diversity among
clinical trial participants and investigators. We also pledged
to invest USD 250 million in R&D for the treatment of
malaria and neglected tropical diseases, building on our
decades-long commitment to global health priorities. We
continue to make progress in other aspects of our ESG
agenda, including reducing greenhouse gas emissions
from our own operations by nearly half since 2016.
As we look to the future as a focused medicines company,
our dedication to innovation and excellence will drive us
forward. We have set clear growth ambitions and we are
confident we will meet them. Through reshaping Novartis,
we are set to reimagine medicine for decades to come.
Sincerely,
Vas Narasimhan
Chief Executive Ocer

2
Table of contents
Table of contents
Introduction and use of certain terms .................................................................................................................................................................
Forward-looking statements ...................................................................................................................................................................................
PART I 7
Item . Identity of Directors, Senior Management and Advisers ...................................................................................................
Item . Oer Statistics and Expected Timetable ...................................................................................................................................
Item . Key Information ........................................................................................................................................................................................
.A [Reserved] ..................................................................................................................................................................................................
.B Capitalization and indebtedness .....................................................................................................................................................
.C Reasons for the oer and use of proceeds ..............................................................................................................................
.D Risk factors ................................................................................................................................................................................................
Item . Information on the Company ..........................................................................................................................................................
.A History and development of Novartis ........................................................................................................................................
.B Business overview ...............................................................................................................................................................................
Innovative Medicines ..........................................................................................................................................................................
Sandoz ......................................................................................................................................................................................................
.C Organizational structure ...................................................................................................................................................................
.D Property, plants and equipment ...................................................................................................................................................
Item A. Unresolved Sta Comments .........................................................................................................................................................
Item . Operating and Financial Review and Prospects ..................................................................................................................
.A Operating results..................................................................................................................................................................................
.B Liquidity and capital resources .....................................................................................................................................................
.C Research and development, patents and licenses .............................................................................................................
.D Trend information .................................................................................................................................................................................
.E Critical accounting estimates ........................................................................................................................................................
Item . Directors, Senior Management and Employees ..................................................................................................................
.A Directors and senior management .............................................................................................................................................
.B Compensation .......................................................................................................................................................................................
.C Board practices..................................................................................................................................................................................
.D Employees ............................................................................................................................................................................................
.E Share ownership................................................................................................................................................................................
Item . Major Shareholders and Related Party Transactions ....................................................................................................
.A Major shareholders ..........................................................................................................................................................................
.B Related party transactions ...........................................................................................................................................................
.C Interests of experts and counsel ..............................................................................................................................................
Item . Financial Information .......................................................................................................................................................................
.A Consolidated statements and other financial information ...........................................................................................
.B Significant changes .........................................................................................................................................................................
Item . The Oer and Listing ......................................................................................................................................................................
.A Oer and listing details ..................................................................................................................................................................
.B Plan of distribution ............................................................................................................................................................................
.C Markets ...................................................................................................................................................................................................
.D Selling shareholders ........................................................................................................................................................................
.E Dilution ....................................................................................................................................................................................................
.F Expenses of the issue ....................................................................................................................................................................
Item . Additional Information .....................................................................................................................................................................
.A Share capital ........................................................................................................................................................................................
.B Memorandum and articles of association ............................................................................................................................
.C Material contracts .............................................................................................................................................................................
.D Exchange controls............................................................................................................................................................................
.E Taxation ..................................................................................................................................................................................................
.F Dividends and paying agents ......................................................................................................................................................
.G Statement by experts .....................................................................................................................................................................
* “Item 5. Operating and Financial Review and Prospects,” together with the sections on compounds in development and selected development projects of our divisions
(see“Item 4. Information on the Company—Item 4.B Business overview”), constitute the Operating and Financial Review (“Lagebericht”), as defined by the Swiss Code of
Obligations.
*
*

3
Table of contents
.H Documents on display ....................................................................................................................................................................
.I Subsidiary information ....................................................................................................................................................................
Item . Quantitative and Qualitative Disclosures About Market Risk ....................................................................................
Item . Description of Securities Other Than Equity Securities...............................................................................................
.A Debt securities ...................................................................................................................................................................................
.B Warrants and rights..........................................................................................................................................................................
.C Other securities .................................................................................................................................................................................
.D American Depositary Shares ......................................................................................................................................................
PART II 176
Item . Defaults, Dividend Arrearages and Delinquencies ..........................................................................................................
Item . Material Modifications to the Rights of Security Holders and Use of Proceeds .............................................
Item . Controls and Procedures ..............................................................................................................................................................
Item A. Audit Committee Financial Expert ...........................................................................................................................................
Item B. Code of Ethics ....................................................................................................................................................................................
Item C. Principal Accountant Fees and Services ..............................................................................................................................
Item D. Exemptions from the Listing Standards for Audit Committees ................................................................................
Item E. Purchases of Equity Securities by the Issuer and Aliated Purchasers .............................................................
Item F. Change in Registrant’s Certifying Accountant ..................................................................................................................
Item G. Corporate Governance ..................................................................................................................................................................
Item H. Mine Safety Disclosure ..................................................................................................................................................................
Item I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections .................................................................
PART III 188
Item . Financial Statements.......................................................................................................................................................................
Item . Financial Statements.......................................................................................................................................................................
Item . Exhibits ...................................................................................................................................................................................................

4
Introduction and use of certain terms
Introduction and use of certain terms
Novartis AG and its consolidated aliates publish consolidated financial statements expressed in US dollars. Our
consolidated financial statements responsive to Item 18 of this Annual Report on Form20-F (Annual Report) are
prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International
Accounting Standards Board (IASB). “Item 5. Operating and Financial Review and Prospects,” together with the
sections on products in development and key development projects of our businesses (see “Item 4. Information on
the Company—Item 4.B. Business overview”), constitute the Operating and Financial Review (“Lagebericht”), as
defined by the Swiss Code of Obligations.
Unless the context requires otherwise, the words “we,” “our,” “us,” “Novartis,” “Group,” “Company,” and similar
words or phrases in this Annual Report refer to Novartis AG and its consolidated aliates. However, each Group
company is legally separate from all other Group companies and manages its business independently through its
respective board of directors or similar supervisory body or other top local management body, if applicable. Each
executive identified in this Annual Report reports directly to other executives of the Group company that employs
the executive, or to that Group company’s board of directors.
In this Annual Report, references to “US dollars,” “USD” or “$” are to the lawful currency of the United States of
America, references to “CHF” are to Swiss francs, and references to “euro” or “EUR” are to the lawful currency of
27 member states participating in the European Union; references to the “United States” or to “US” are to the United
States of America, references to the “European Union” or to “EU” are to the European Union and its 27 member
states, references to “Latin America” are to Central and South America, including the Caribbean, and references
to “Australasia” are to Australia, New Zealand, Melanesia, Micronesia and Polynesia, unless the context otherwise
requires; references to the “EC” are to the European Commission; references to “associates” are to employees of
our aliates; references to the “SEC” are to the US Securities and Exchange Commission; references to the “FDA”
are to the US Food and Drug Administration; references to the “EMA” are to the European Medicines Agency, an
agency of the EU, and references to the “CHMP” are to the Committee for Medicinal Products for Human Use of
the EMA; references to “ADR” or “ADRs” are to Novartis American Depositary Receipts, and references to “ADS”
or “ADSs” are to Novartis American Depositary Shares; references to the “NYSE” are to the New York Stock
Exchange, and references to “SIX” are to the SIX Swiss Exchange; references to “ECN” are to the Executive Com-
mittee of Novartis; references to “GSK” are to GlaxoSmithKline plc; references to “Roche” are to Roche Holding
AG; references to “Gyroscope Therapeutics” are to Gyroscope Therapeutics Holdings plc; references to “AAA” are
to Advanced Accelerator Applications S.A., references to “Novartis Gene Therapies” are to Novartis Gene Thera-
pies, Inc., and references to “Endocyte” are to Endocyte, Inc.
All product names appearing in italics are trademarks owned by or licensed to Group companies. Product names
identified by a “
®
” or a “™” are trademarks that are not owned by or licensed to Group companies and are the prop-
erty of their respective owners.

5
Forward-looking statements
Forward-looking statements
This Annual Report contains certain forward-looking statements within the meaning of Section 27A of the Securi-
ties Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), and the United States Private Securities Litigation Reform Act of 1995, as amended. Other written materials
filed with or furnished to the SEC by Novartis, as well as other written and oral statements made to the public, may
also contain forward-looking statements. Forward-looking statements can be identified by words such as “poten-
tial,” “expected,” “will,” “planned,” “pipeline,” “outlook,” “may,” “could,” “would,” “anticipate,” “seek,” or similar terms,
or by express or implied discussions regarding potential new products, potential new indications for existing prod-
ucts, or regarding potential future revenues from any such products; or regarding the potential outcome, or finan-
cial or other impact on Novartis, of any of the transactions described; or regarding the potential impact of share
buybacks; or regarding potential future sales or earnings of the Group or any of its divisions or potential share-
holder returns; or regarding potential future credit ratings of the Group; or by discussions of strategy, plans, expec-
tations or intentions. Such forward-looking statements are based on the current beliefs and expectations of man-
agement regarding future events, and are subject to significant known and unknown risks and uncertainties. Should
one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual
results may vary materially from those set forth in the forward-looking statements. You should not place undue reli-
ance on these statements.
In particular, our expectations could be aected by, among other things:
• Uncertainties regarding the success of key products and commercial priorities;
• Uncertainties in the research and development of new healthcare products, including clinical trial results and
additional analysis of existing clinical data;
• Global trends toward healthcare cost-containment, including ongoing government, payer and general public pric-
ing and reimbursement pressures and requirements for increased pricing transparency;
• The potential that the strategic benefits, operational eciencies or opportunities expected from our external busi-
ness opportunities, our intention to separate our Sandoz Division into a new publicly traded standalone company
by way of a 100% spin-o, or the implementation of our new organizational structure and operating model, may
not be realized or may take longer to realize than expected;
• Our ability to obtain or maintain proprietary intellectual property protection, including the ultimate extent of the
impact on Novartis of the loss of patent protection and exclusivity on key products that commenced in prior years
and is expected to continue this year;
• Our performance on environmental, social and governance matters;
• Uncertainties in the development or adoption of potentially transformational digital technologies and business
models;
• Uncertainties regarding potential significant breaches of information security or disruptions of our information
technology systems;
• Uncertainties surrounding the implementation of our new Enterprise Resource Planning system and other IT proj-
ects;
• Our reliance on outsourcing key business functions to third parties;
• Uncertainties regarding actual or potential legal proceedings, including, among others, litigation and other legal
disputes with respect to our recent transactions, product liability litigation, litigation and investigations regarding
sales and marketing practices, intellectual property disputes and government investigations generally;
• Safety, quality, data integrity or manufacturing issues;
• Our ability to attract, integrate and retain key personnel and qualified individuals;
• Regulatory actions or delays or government regulation generally, including potential regulatory actions or delays
with respect to the development of the products described in this Annual Report;

6
Forward-looking statements
• Our ability to comply with data privacy laws and regulations, and uncertainties regarding potential significant
breaches of data privacy;
• Our ability to adapt to major geopolitical and macroeconomic developments, including the eects of and eorts
to mitigate pandemic diseases such as COVID-19, and the impact of the war in Ukraine;
• Uncertainties involved in predicting shareholder returns;
• Uncertainties regarding the eects of recent and anticipated future changes in tax laws and their application to
us;
• Uncertainties regarding future global exchange rates; and
• Uncertainties regarding future demand for our products.
Some of these factors are discussed in more detail in this Annual Report, including under “Item 3. Key Information—
Item 3.D. Risk factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and
Prospects.” Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove
incorrect, actual results may vary materially from those described in this Annual Report as anticipated, believed,
estimated or expected. We provide the information in this Annual Report as of the date of its filing. We do not intend,
and do not assume any obligation, to update any information or forward-looking statements set out in this Annual
Report as a result of new information, future events or otherwise.

Item 1. Identity of Directors, SeniorManagement and Advisers
7
PART I
Item 1. Identity of Directors,
SeniorManagement and Advisers
Not applicable.

Item 2. Oer Statistics and Expected Timetable
8
Item 2. Oer Statistics and Expected
Timetable
Not applicable.

Item 3. Key Information
9
Item 3. Key Information
3.A [Reserved]
3.B Capitalization and indebtedness
Not applicable.
3.C Reasons for the oer and use of proceeds
Not applicable.
3.D Risk factors
Our businesses face significant risks and uncertainties.
You should carefully consider all of the information set
forth in this Annual Report and in other documents we
file with or furnish to the SEC, including the following risk
factors, before deciding to invest in or to maintain an
investment in any Novartis securities. Our business, as
well as our reputation, financial condition, results of oper-
ations, and share price, could be materially adversely
aected by any of these risks, as well as other risks and
uncertainties not currently known to us or not currently
considered material.
Strategic risks
Key products and commercial priorities
Risk description
Failure to deliver key commercial priorities and success-
fully launch new products
Context and potential impact
Our ability to maintain and grow our business and to
replace revenue and income lost to generic, biosimilar
and other competition depends heavily on the commer-
cial success of our new or existing key products. The
commercial success of these products could be impacted
at any time by a number of factors, including pressure
from new or existing competitive products, changes in
the prescribing habits of healthcare professionals, unex
-
pected side eects or safety signals, supply chain issues
or other product shortages, pricing pressure, regulatory
proceedings, changes in labeling, loss of intellectual
property protection, and global pandemics. In addition,
our revenue and margins could be significantly impacted
by the timing and rate of commercial acceptance of new
products.
Healthcare professionals, patients and payers may
choose competitor products instead of ours for various
reasons, including if they perceive them to be better in
terms of ecacy, safety, cost, convenience or other rea-
sons. The commercial success of our key products and
launches in the face of increasing competition requires
significant attention and management focus. Such com-
petition could significantly aect the revenue from our
products and our results of operations. This impact could
also be compounded to the extent that such competi-
tion results in us making significant additional invest-
ments in research and development, marketing or sales.
Research and development
Risk description
Failure to successfully prioritize, integrate and execute
our research and development programs for new prod-
ucts or new indications for existing products, given our
focus on innovative medicines
Context and potential impact
We engage in extensive and costly research and devel-
opment activities, both through our own internal
resources and through collaborations with third parties,
in an eort to identify and develop new products and
new indications for existing products that address unmet
and changing medical needs, and that are commercially
successful. Our ability to grow our business and our
product pipeline; to replace sales lost due to branded
competition, entry of generics, or other reasons; and to
bring to market products that take advantage of new and
potentially disruptive technologies, including cell, gene
and radioligand therapies, depends in significant part on
the success of these eorts.
Failure to successfully develop our pipeline products
is typically the result of the inherent uncertainty of sci-
ence, suboptimal internal execution, or both. Key ele-
ments of internal execution include our ability to priori-
tize our investments on our highest potential value assets,
optimize the transition of assets from research to

Item 3. Key Information
10
development, integrate externally acquired assets in an
ecient way, and execute the steps in our drug develop-
ment process that enable our assets to be approved and
reimbursed in a timely manner to positively impact clin-
ical practice. See also “Item 4. Information on the Com-
pany— Item 4.B Business overview—Innovative
Medicines—Research and development” with regards to
the research and development eorts of our Innovative
Medicines Division.
Our new products must undergo intensive preclinical
and clinical testing and are approved by means of a highly
complex, lengthy, and expensive approval process that
varies substantially from country to country and may
have very specific requirements for the recruitment of
patients for clinical trials. We face increasing and evolv-
ing regulatory approval and reimbursement require-
ments. If we fail to successfully progress late-stage
assets and the core elements of drug development for
key programs, this could have a negative impact on the
development of our product pipeline, and ultimately on
the success of our business and our financial results.
In addition, in the US it is becoming increasingly chal-
lenging to adequately recruit a sucient number of US
patients in clinical trials due to new and changing require-
ments for recruitment of patients into such trials. As a
result, we may be unable to develop the necessary clin-
ical evidence to support the desired indications and
product profile for a particular disease that is needed to
drive clinical adoption of our new products, and thereby
achieve the full potential of our assets (also known as
the “target product profile”). Similarly, the post-approval
regulatory burden has also increased. These require-
ments make the maintenance of regulatory approvals for
our products increasingly expensive, and further heighten
the risk of recalls, product withdrawals, change to prod-
uct specifications, loss of market share, and loss of rev-
enue and profitability.
The clinical testing, regulatory processes and
post-approval activities described above become more
dicult during pandemics, such as the COVID-19 pan-
demic, as well as during periods of geopolitical and eco-
nomic uncertainty. This is due to challenges related to
recruiting, enrolling and treating patients in clinical trials,
as well as ensuring the supply of trial materials. For a fur-
ther description of the research and development of, and
approval processes for, the products of our Innovative
Medicines Division, see the sections headed “Research
and development” and “Regulation” included in the
description of our Innovative Medicines Division under
“Item 4. Information on the Company—Item 4.B Business
overview—Innovative Medicines.”
Our Sandoz Division has made, and expects to
continue to make, significant investments in the devel-
opment of biotechnology-based, “biologic” medicines
that are intended for sale as bioequivalent or “biosimilar”
versions of currently marketed biotechnology products.
While the development of such products is typically
significantly less costly and complex than the develop-
ment of the equivalent originator medicines, it is
nonetheless significantly more costly and complex than
that for typical small-molecule generic products.
Formore information about the research and develop-
ment eorts of our Sandoz Division, see “Item 4.
Information on the Company—Item 4.B Business
overview— Sandoz—Development and registration.” In
addition, many countries do not yet have fully developed
legislative or regulatory pathways to facilitate the devel-
opment of biosimilars, and to permit their sale in such a
way that they are readily substitutable alternatives to the
originator product. Further delays or diculties in the
development or marketing of biosimilars could put at risk
the significant investments that Sandoz has made, and
will continue to make, in its Biopharmaceuticals business.
Failure to successfully develop and market biosimilars
could have a material adverse eect on the success of
the Sandoz Division and the Group as a whole. For more
information about the approval processes that must be
followed to market Sandoz Division products, see “Item
4. Information on the Company—Item 4.B Business over-
view—Sandoz—Regulation.”
Furthermore, our research and development activi-
ties must be conducted in an ethical and compliant man-
ner. Among other things, we are concerned with patient
safety (both pre- and post-product approval), data pri-
vacy, current Good Clinical Practices (cGCP) require-
ments, data integrity, the fair treatment of patients, diver-
sity and inclusion in the recruitment of patients to clinical
trials, and animal welfare. Should we fail to properly man-
age such issues, we risk injury to third parties, damage
to our reputation, negative financial consequences as a
result of potential claims for damages, sanctions and
fines, and the potential that investments in research and
development activities may not bring the expected ben-
efits to the Group.
Pricing, reimbursement and access
Risk description
Pricing and reimbursement pressure, including pricing
transparency and access to healthcare
Context and potential impact
Our business has continuously experienced significant
pressures on the pricing of our products and on our abil-
ity to obtain and maintain satisfactory rates of reimburse-
ment for our products by governments, insurers and
other payers. These pressures have many sources,
including growth of healthcare costs as a percentage of
gross domestic product; funding restrictions and policy
changes; and public controversies, political debate,
investigations and legal proceedings regarding pharma-
ceutical pricing. Pressures on pricing may negatively
impact both our product pricing and the availability of
our products.
In addition, we face numerous cost-containment
measures imposed by governments and other payers.
These include government-imposed industrywide price
reductions, mandatory pricing systems, reference pric-
ing systems, payers limiting access to treatments based
on cost-benefit analyses, the importation of drugs from
lower-cost countries to higher-cost countries, the shift-
ing of the payment burden to patients through higher
co-payments and co-pay accumulator programs, the lim
-
iting of physicians’ ability to choose among competing
medicines, the mandatory substitution of generic drugs
for the patented equivalent, pressure on physicians to
reduce the prescribing of patented prescription
medicines, increasing pressure on intellectual property

Item 3. Key Information
11
protections, and growing requirements for increased
transparency on pricing. For more information on price
controls, see “Item 4. Information on the Company—Item
4.B Business overview—Innovative Medicines—Price
controls.”
Recent trends in the external environment may have
an impact on the likelihood of these pricing and reim-
bursement pressures occurring. A worldwide slowdown
in economic growth following the COVID-19 pandemic
and the war in Ukraine (contributing to challenges such
as high energy costs and inflation) has led to increased
strain on fiscal budgets in many major economies. In
addition, legislative developments such as those in the
US (e.g., the Inflation Reduction Act) and in Europe (e.g.,
the EU Joint Health Technology Assessment) pose
potential further pressures on pricing and timelines for
reimbursement in these countries. These external fac-
tors may materially aect our ability to achieve val-
ue-based prices; to achieve and maintain an acceptable
return on our investments in the research and develop-
ment of our products; and may impact our ability to
research and develop new products.
In addition, our Sandoz Division has faced and may
continue to face intense competition from other generic
and biosimilar pharmaceutical companies that aggres-
sively compete for market share, including through sig-
nificant price competition. Such competitive actions may
increase the costs and risks associated with our eorts
to introduce and market generic and biosimilar products,
may delay the introduction or marketing of such prod-
ucts, and may further limit the prices at which we are
able to sell these products. In particular, in the US in past
years, industrywide price competition among generic
pharmaceutical companies and consolidation of buyers
caused significant declines in sales and profits of Sandoz.
Alliances, acquisitions and divestments
Risk description
Failure to identify, execute, and/or realize the expected
benefits from our external business opportunities
Context and potential impact
As part of our strategy, from time to time we acquire and
divest products or entire businesses and enter into stra-
tegic alliances and collaborations. For example, in Feb-
ruary 2022, we closed the acquisition of Gyroscope
Therapeutics. This strategy is partly dependent on our
ability to identify strategic external business opportuni-
ties and to close transactions with third parties on
acceptable terms.
Once the terms of a strategic transaction have been
agreed with a third party, we may not be able to com-
plete the transaction in a timely manner or at all, nor can
we be sure that pre-transaction due diligence will iden-
tify all possible issues that might arise during and after
the transaction. Our eorts on such transactions can
also divert management’s attention from our existing
businesses.
After a transaction is closed, eorts to develop and
market acquired or licensed products, to integrate the
acquired business or to achieve expected synergies may
fail or may not fully meet expectations. This may occur
due to diculties in retaining key personnel, customers
and suppliers; failure to obtain marketing approval or
reimbursement within expected time frames or at all; dif-
ferences in corporate culture, standards, controls, pro-
cesses and policies; or other factors. Transactions can
also result in liabilities being incurred that were not
known at the time of acquisition, or the creation of tax
or accounting issues. Acquired businesses are not
always in full compliance with legal, regulatory or Novartis
standards, including, for example, Current Good Manu-
facturing Practices (cGMP) or cGCP standards, which
can be costly and time-consuming to remediate. Further-
more, our strategic alliances and collaborations with third
parties may not achieve their intended goals and objec-
tives within expected time frames, or at all.
Similarly, we cannot ensure that we will be able to
successfully divest or spin o businesses or other assets
that we have identified for this purpose, or that any com-
pleted divestment or spin-o will achieve the expected
strategic benefits, operational eciencies or opportuni-
ties, or that the divestment or spin-o will ultimately max-
imize shareholder value.
Intellectual property
Risk description
Expiry, assertion or loss of intellectual property protec-
tion
Context and potential impact
Many products of our Innovative Medicines Division are
protected by intellectual property rights, which may pro-
vide us with exclusive rights to market those products
for a limited time, and to enable our purpose of reimag-
ining medicine by sustainably financing our research and
development. However, the strength and duration of
those rights can vary significantly from product to prod-
uct and from country to country, and they may be suc-
cessfully challenged by third parties or governmental
authorities.
Loss of intellectual property protection and the intro-
duction of generic or biosimilar competition for a pat-
ented branded medicine in a country typically result in a
significant and rapid reduction in net sales and operat-
ing income for the branded product. Such competition
can occur after successful challenges to intellectual
property rights or the regular expiration of the patent
term or other intellectual property rights. Such compe-
tition can also result from the entry of generic or biosim-
ilar versions of another medicine in the same therapeu-
tic class as one of our drugs or in a competing
therapeutic class, from a Declaration of Public Interest
or the compulsory licensing of our intellectual property
by governmental authorities, or as a result of a general
weakening of intellectual property and governing laws in
certain countries around the world. In addition, generic
or biosimilar manufacturers may sometimes conduct
so-called “launches at risk” of products that are still
under legal challenge for infringement, or whose patents
are still under legal challenge for validity, before final res-
olution of legal proceedings.
We also rely in all aspects of our businesses on unpat-
ented proprietary technology, know-how, trade secrets,

Item 3. Key Information
12
and other confidential information, which we seek to pro-
tect through various measures, including confidentiality
agreements with licensees, employees, third-party col-
laborators and consultants who may have had access to
such information. If these agreements are breached or
our other protective measures should fail, then our con-
tractual or other remedies may not be adequate to cover
our losses.
We may also be subject to assertions of intellectual
property rights against our innovative medicines by third
parties. If successful, these actions may involve payment
of future royalties or damages, for example for patent
infringement, and may also involve injunctive relief requir-
ing the removal of one or more dosage strengths of a
product from the market (or removal of a therapeutic
indication from the product’s approved labeling) for some
period of time or throughout the life of the asserted intel-
lectual property right. Such damages or such an injunc-
tion may have a material impact on our operating income
and net sales.
In any given year, we may experience a potentially
significant impact on our net sales from products that
have already lost intellectual property protections, as
well as products that may lose protection during the year.
Because we may have substantially reduced marketing
and research and development expenses related to
products that are in their final years of exclusivity, the
initial loss of protection for a product during a given year
could also have an impact on our operating income for
that year in an amount corresponding to a significant
portion of the product’s lost sales. The magnitude of the
impact of generic or biosimilar competition on our income
could depend on a number of factors. These include,
with respect to income in a given year, the time of year
at which the generic or biosimilar competitor is launched;
the ease or diculty of manufacturing a competitor prod
-
uct and obtaining regulatory approval to market it; the
number of generic or biosimilar competitor products
approved, including whether, in the US, a single compet-
itor is granted an exclusive marketing period; whether an
authorized generic is launched; the geographies in which
generic or biosimilar competitor products are approved,
including the strength of the market for generic or bio-
similar pharmaceutical products in such geographies,
and the comparative profitability of branded pharmaceu-
tical products in such geographies; and our ability to suc-
cessfully develop and launch new products for patients
that may also oset the income lost to generic or bio-
similar competition. For more information on the patent
and generic competition status of our Innovative
Medicines Division products, see “Item 4. Information on
the Company—Item 4.B Business overview—Innovative
Medicines—Intellectual property.”
Strategic transformations
Risk description
Failure to meet organizational transformation programs
objectives and/or unintended adverse impacts on our
business
Context and potential impact
From time to time, we reassess our business organiza-
tion to ensure we have the optimal structure with which
to execute our strategy. In April 2022, we announced a
new organizational structure and operating model
designed to support our innovation, growth, and produc-
tivity ambitions as a focused medicines company. See
“Item 4. Information on the Company—Item 4.B Over-
view.”
In addition, in October 2021 we announced the com-
mencement of a strategic review of our Sandoz Division.
After exploring all options, ranging from retaining the
business to separation, on August 25, 2022, we
announced our intention to separate our Sandoz Division
into a new publicly traded standalone company, by way
of a 100% spin-o in order to maximize shareholder
value. See “Item 4. Information on the Company—Item
4.B Sandoz.”
Our inability to successfully implement our new orga
-
nizational structure and operating model or to success-
fully complete the spin-o of our Sandoz Division could
have a material adverse eect on the success of the
Group as a whole, and could have a material adverse
eect on our results of operations and financial condi-
tion. The overall extent and pace of these organizational
changes, and the additional workload and complexity for
our employees in some areas, could trigger uncertainty,
stress and fatigue among employees, potentially result-
ing in instability within the organization that could lead
to failure of these organizational changes to succeed or
to achieve the desired benefits. As a result, the expected
benefits of these organizational changes may never be
fully realized or may take longer to realize than expected.
Environmental, social and governance matters
Risk description
Failure to meet environmental, social and governance
expectations
Context and potential impact
Increasingly, in addition to financial results, companies
are being judged by performance on a variety of envi-
ronmental, social and governance (ESG) matters, which
can contribute to the long-term sustainability of our com-
pany’s performance. An inability to successfully perform
on ESG matters and to meet societal expectations can
result in negative impacts on our recruitment, retention,
operations, financial results, reputation, and share price.
Topics related to large societal changes such as
social inequity, access to medicines and climate change
are increasingly important to a wide range of our stake-
holders. For example, a variety of organizations measure
the performance of companies on ESG topics, and the
results of these assessments are widely publicized. In
addition, investments in funds that specialize in compa-
nies that perform well in such assessments are increas-
ingly popular, and major institutional investors have pub-
licly emphasized the importance of such ESG measures
in making their investment decisions. Our actions related

Item 3. Key Information
13
to ESG topics may in the long-term therefore impact our
operations and ability to achieve our strategic goals, and
ultimately could have a potential negative impact on the
value of Novartis. For this reason, the role of our Board
of Directors and executive ocers in supervising various
sustainability issues is becoming increasingly important.
We actively manage a broad range of ESG matters,
taking into consideration their expected impact on the
sustainability of our business over time, and the poten-
tial impact of our business on society and the environ-
ment. We have created a Sustainability & ESG Oce,
which, in coordination with the ESG Committee of the
Executive Committee of Novartis, is tasked with devel-
oping our ESG strategy and tracking our performance
against our ESG targets. However, considering investors’
increasing focus on ESG matters, the fast pace of change
of external expectations, and a range of upcoming reg-
ulations, there can be no certainty that we will manage
such issues successfully, that the ESG standards we cur
-
rently use to measure our performance against will
remain the same, or that we will successfully meet soci-
ety or investors’ expectations.
Operational risks
Cybersecurity and IT systems
Risk description
Cybersecurity breaches, data loss and catastrophic loss
of IT systems
Context and potential impact
We are heavily dependent on critical, complex and inter-
dependent information technology (IT) systems, includ-
ing internet-based systems to support our business pro-
cesses. We have also outsourced significant parts of our
IT infrastructure to third-party providers, and we cur-
rently use these providers to perform business-critical
IT services for us. We are therefore vulnerable to cyber-
security attacks and incidents on such networks and
systems, whether our own or those of the third-party
providers we contract, and we have experienced and
may in the future experience such cybersecurity threats
and attacks. Cybersecurity threats and attacks take
many forms, and the size, age and complexity of our IT
systems make them potentially vulnerable to external
and internal security threats; outages; malicious intru-
sions and attacks; cybercrimes, including state-spon-
sored cybercrimes; malware; misplaced data, lost data
or data errors; programming or human errors; or other
similar events. In the context of the COVID-19 pandemic,
the risk of such threats and attacks has increased, as
virtual and remote working has become more widely
used, and sensitive data is accessed by employees work-
ing in less secure, home-based environments. In addi-
tion, due to our reliance on third-party providers, we have
experienced and may in the future experience interrup-
tions, delays or outages in IT service availability due to
a variety of factors outside of our control, including tech-
nical failures, natural disasters, fraud, or security attacks
experienced by or caused by third-party providers. Inter-
ruptions in the service provided by these third parties
could aect our ability to perform critical tasks.
A significant information security or other event, such
as a disruption or loss of availability of one or more of
our IT systems, whether managed by us or a third-party
service provider, has previously and could in the future
negatively impact important business processes, such
as the conduct of scientific research and clinical trials,
the submission of data and information to health author-
ities, our manufacturing and supply chain processes, our
shipments to customers, our compliance with legal obli-
gations, and communication between employees and
with third parties. IT issues have previously led to, and
could in the future lead to, the compromise of trade
secrets or other intellectual property that could be sold
and used by competitors to accelerate the development
or manufacturing of competing products; to the compro-
mise of personal financial and health information; and to
the compromise of IT security data such as usernames,
passwords and encryption keys, as well as security strat-
egies and information about network infrastructure,
which could allow unauthorized parties to gain access
to additional systems or data. In addition, malfunctions
in software or medical devices that make significant use
of IT could lead to a risk of direct harm to patients.
Although we have experienced some of the events
described above, to date they have not had a material
impact on our operations. Nonetheless, the occurrence
of any of the events described above in the future could
disrupt our business operations and result in enforce-
ment actions or liability, including potential government
fines and penalties, claims for damages, and shareholder
litigation or allegations that the public health, or the
health of individuals, has been harmed.
Any significant events of this type could require us to
expend significant resources beyond those we already
invest to remediate any damage, to further modify or
enhance our protective measures, and to enable the con-
tinuity of our business.
Fragmented IT landscape and strategic technology
programs implementation
Risk description
Failure to address fragmented business processes,
unclear data ownership, and IT applications and infra-
structure nearing their end-of-life, may disrupt our core
business processes
Context and potential impact
We rely on various IT systems to operate our complex
global business. Historically, while highly overlapping
data strategy and architectural needs exist across our
businesses, in the past we built distinct solutions across
both prior business units and our various geographies,
which have led to a fragmented and complex landscape
of IT systems. Additionally, several of our current IT sys-
tems are reaching the end of their useful life, which,
together with our fragmented IT landscape, may cause
disruptions to our operational stability. As a result, we
started to implement several companywide IT programs
with a view toward replacing and consolidating outdated
IT systems. For example, we have completed the con-
ceptual design phase and started to build a new global
Enterprise Resource Planning (ERP) system that seeks
to simplify, standardize and digitize processes in our

Item 3. Key Information
14
commercial, finance and operations functions, thereby
helping to ensure ecient and compliant business oper-
ations across our businesses and geographies, as well
as the availability of high-quality data necessary to aid
our decision-making. We expect the first implementation
of our new ERP system to begin in the first quarter of
2024, with full implementation by 2028. In addition, we
are also implementing other IT projects, seeking to sim-
plify and standardize our processes, systems and tools,
and create a unified data marketplace. Implementation
and operation of the new ERP system and other IT proj-
ects involves certain risks, including a failure of the new
ERP system and other IT projects to operate as expected,
a failure to properly integrate with other systems we use,
potential loss of data or information, compliance issues,
or cost overruns and delays. Any disruptions or malfunc-
tions of the new ERP system and other IT projects could
cause critical information to be delayed, lost, defective,
corrupted, or rendered inadequate or inaccessible,
which could negatively impact our operations and the
eectiveness of our internal controls.
Talent management
Risk description
Inability to attract, retain and motivate qualified individ-
uals in key roles and markets
Context and potential impact
We rely on attracting and retaining a diverse, highly
skilled workforce across our businesses and functions
to achieve our business objectives. If we are unable to
sustain our supply of key personnel – including senior
members of our scientific and management teams,
high-quality researchers and development specialists
and skilled employees in key markets – our ability to
achieve our major business objectives may be adversely
aected. In addition, our brand and reputation could be
negatively impacted, and the diversity of our workforce
may decline.
The market for skilled talent has become increasingly
competitive, and we anticipate this trend will persist long-
term. We face a challenge to attract and retain top tal-
ent in several areas, including biology, chemistry, clinical
development, drug manufacturing, IT, oncology, and
advanced therapy platforms (i.e., gene and cell therapy,
radioligand therapy and “xRNA”). In addition, many bio-
technology companies have received significant inflows
of capital and are not only competing with us to attract
the same skilled talent but are also aggressively pursu-
ing our experienced talent.
In recent years, we have adopted new ways of work-
ing that include location flexibility and increasingly
recruiting from a global pool of talent. However, the suc-
cess of our business continues to depend on having
employees who possess local knowledge of, and expe-
rience in, our key markets. The external talent supply is
especially limited in many of the geographies that are
expected to be sources of growth for Novartis. In the
United States, China and several other markets, the geo-
graphic mobility of talent is decreasing, as they find
ample career opportunities available closer to home.
In addition, in April 2022 we announced a new, inte-
grated organizational structure and operating model. The
corporate reorganization undertaken to implement this
new organizational structure has resulted in significant
redundancies and senior leadership changes that may
reduce morale, increase employee distraction and
prompt higher voluntary turnover, any of which could
negatively impact our competitiveness and ability to
achieve strategic objectives. For more information on
this new organizational structure see “Item 4. Informa-
tion on the Company—Item 4.B Overview.”
The risks associated with the challenging external
talent market and the implementation of our new orga-
nizational structure will be exacerbated if we are unable
to retain and eectively develop employees and main-
tain an internal pipeline with critical skills, experiences,
and leadership to deliver our business priorities. As a
result, development, engagement, motivation, succes-
sion planning and performance rewards for our critical
talent are essential to achieve our business priorities.
Third-party management
Risk description
Failure to maintain adequate governance and oversight
over third-party relationships, and failure of third parties
to meet their contractual, regulatory or other obligations
Context and potential impact
We outsource the performance of certain key business
functions and services to third parties. Such activities
include research and development collaborations, man-
ufacturing operations, warehousing and distribution, cer-
tain finance functions, sales and marketing activities,
data management and others. Some third parties, par-
ticularly those in developing countries, do not have inter-
nal compliance systems or resources comparable to
those of Novartis. As a result, our investment and eorts
in relation to third party management include focusing
on risk management and the oversight of such third par-
ties.
Our reliance on third parties poses certain risks,
including the misappropriation of our intellectual prop-
erty, the failure of the third party to comply with regula-
tory and quality assurance requirements, the failure of
the third party to comply with environmental, anti-brib-
ery and human rights standards and regulations, unex-
pected supply disruptions, breach of our agreement by
the third party, and the unexpected termination or non-
renewal of our agreement by the third party.
In addition, governments require, and the public
expects, Novartis to take responsibility for and report on
compliance with various human rights, responsible
sourcing and environmental practices, as well as other
actions of our third-party contractors around the world.
Ultimately, if third parties fail to meet their obligations
to us, we may lose our investment in the relationship with
the third parties or fail to receive the expected benefits
of our agreements with such third parties. In addition,
should any of these third parties fail to comply with the
law or our standards, or should they otherwise act inap-
propriately while performing services for us, there is a
risk that we could be held responsible for their acts, that
our reputation may suer, and that penalties may be
imposed on us.

Item 3. Key Information
15
Legal, ethics and compliance
Risk description
Challenges posed by evolving legal and regulatory
requirements and societal expectations regarding ethi-
cal behavior
Context and potential impact
We must comply with the laws of all countries in which
we operate, and we sell products with respect to a wide
and growing range of activities. Such legal requirements
are extensive and complex.
The laws and regulations relevant to the healthcare
industry and applicable to us are broad in scope, are sub
-
ject to change, and have evolving interpretations, which
could require us to incur substantial costs associated
with compliance or to alter one or more of our business
practices. For example, we have been, are currently, and
may in the future be, subject to various significant legal
proceedings, such as private party litigation, government
investigations and law enforcement actions worldwide.
These types of matters may take various forms based
on evolving government enforcement and private party
litigation priorities, and could include, for example, mat-
ters pertaining to pricing; bribery and corruption; trade
regulation and embargo legislation; product liability;
commercial disputes; employment and wrongful dis
-
charge; antitrust and competition; securities; govern-
ment benefit programs; reimbursement; rebates; health-
care fraud; sales and marketing practices; insider trading;
occupational health and safety; environmental regula-
tions; tax; cybersecurity; data privacy; regulatory inter-
actions; and intellectual property. Such matters can
involve civil and/or criminal proceedings and can retro-
actively challenge practices previously considered to be
legal.
There is also a risk that governance for our medical
and patient support activities, and our interactions with
governments, public ocials/institutions, healthcare
professionals, healthcare organizations and patient
organizations may be inadequate or fail, or that we may
undertake activities based on improper or inadequate
scientific justification.
Our Sandoz Division may from time to time seek
approval to market a generic version of a product before
the expiration of patents claimed by the marketer of the
patented product. We do this in cases in which we believe
the relevant patents are invalid or unenforceable, or
would not be infringed by our generic product. As a result,
aliates of our Sandoz Division frequently face patent
litigation, and in certain circumstances we may make the
business decision to market a generic product even
though patent infringement actions are still pending.
Should we elect to do so and conduct a so-called “launch
at risk,” we could face substantial damages if the final
court decision is adverse to us.
Legal proceedings and investigations are inherently
unpredictable, and large judgments sometimes occur.
Consequently, we may in the future incur judgments that
could involve large payments, including the potential
repayment of amounts allegedly obtained improperly,
and other penalties, including treble damages. In addi-
tion, such legal proceedings and investigations, even if
meritless, may aect our reputation, may create a risk of
potential exclusion from government reimbursement
programs in the US and other countries, and may lead
to civil litigation and/or criminal exposure. As a result,
having considered all relevant factors, we have in the
past and may again in the future enter into major settle-
ments of such claims without bringing them to final legal
adjudication by courts or other such bodies, despite hav-
ing potentially significant defenses against them, to limit
the risks they pose to our business and reputation. Such
settlements may require us to pay significant sums of
money and to enter into corporate integrity or similar
agreements, which are intended to regulate company
behavior for extended periods.
For information on significant legal matters pending
against us, see “Item 18. Financial Statements—Note 20.
Provisions and other non-current liabilities” and “Item 18.
Financial Statements—Note 28. Commitments and con-
tingent liabilities.”
New requirements may also be imposed on us due
to changing government and societal expectations
regarding the healthcare industry, and acceptable cor-
porate behavior generally. For example, we are faced
with laws and regulations requiring changes in how we
do business, including with respect to disclosures con-
cerning our interactions with healthcare professionals,
healthcare organizations and patient organizations.
These laws and regulations include requirements that
we disclose payments or other transfers of value made
to healthcare professionals and organizations, as well as
information relating to the costs and prices for our prod-
ucts, which represent evolving standards of acceptable
corporate behavior. These requirements may incur sig-
nificant costs, including substantial time and additional
resources, that are necessary to bring our interactions
with healthcare professionals and organizations into
compliance with these evolving standards.
In addition to legal and regulatory requirements, we
aim to meet the evolving societal expectations of the
public and our investors regarding ethical behavior and
the increasing importance placed on ESG matters.
To support our eorts to comply with the many
requirements that impact us, we have a significant global
ethics and compliance program in place, and we devote
substantial time and resources to eorts to ensure that
we conduct business in a lawful manner, and in line with
society’s expectations. Despite our eorts, an actual or
alleged failure to comply with the law or with heightened
public expectations could lead to substantial liabilities
that may not be covered by insurance, or to other signif-
icant losses.
Manufacturing and product quality
Risk description
Inability to ensure proper controls in product develop-
ment and product manufacturing, and failure to comply
with applicable regulations and standards
Context and potential impact
The development and manufacture of our products is
complex and heavily regulated by governmental health
authorities around the world. Whether or not our prod-
ucts and the related raw materials are developed and
manufactured at our own manufacturing sites or by third

Item 3. Key Information
16
parties, we must ensure that all development and man-
ufacturing processes comply with regulatory require-
ments, as well as our own quality standards in order to
deliver novel therapies to patients with unmet needs
while ensuring patient safety. Failure to comply with reg-
ulatory requirements has resulted in, and may in the
future result in, warning letters, suspension of manufac-
turing, seizure of products, injunctions, product recalls,
failure to secure product approvals, or debarment.
In recent years, global health authorities have sub-
stantially intensified their scrutiny of manufacturers’
compliance with regulatory requirements. Any significant
failure by us or our third-party suppliers to comply with
regulatory requirements, or with health authorities’
expectations, may create the need to suspend clinical
trials, shut down production facilities or production lines,
and recall commercial products. A failure to fully comply
with regulatory requirements could also lead to a delay
in the approval of new products, an inability to ship or
import our products, and significant penalties and repu-
tational harm.
In addition, the technically complex manufacturing
processes required to manufacture many of our prod-
ucts increase the risk of both production failures and
product recalls and can increase the cost of producing
our goods. Some of our products require a supply of
highly specialized raw materials, such as cell lines, tis-
sue samples, bacteria, viral strains and radioisotopes. In
addition, we manufacture and sell a number of sterile
products, biologic products and products that involve
advanced therapy platforms, such as CART therapies,
gene therapies and radioligand therapy products, all of
which are particularly complex and involve highly spe-
cialized manufacturing technologies. As a result, even
slight deviations at any point in their production pro-
cesses or in material used have led to, and may in the
future lead to, production failures or recalls. See “Item
4. Information on the Company—Item 4.B. Business over-
view—Sandoz—Production.”
Supply chain
Risk description
Inability to maintain continuity of product supply
Context and potential impact
Many of our products are produced using technically
complex manufacturing processes and require a supply
of highly specialized raw materials. For some of our prod-
ucts and raw materials, we may rely on a single source
of supply. In addition, we manufacture and sell a number
of sterile products, biologic products, and products that
involve advanced therapy platforms, such as gene and
cell therapy, radioligand therapy, and “xRNA”, all of which
are particularly complex and involve highly specialized
manufacturing technologies. Due to this complexity,
there is a risk of production and supply of critical raw
materials failures, which may result in supply interrup-
tions or product recalls due to manufactured products
not meeting required specifications.
In addition, due to the inherent complexities of our
manufacturing processes and the supply chains for
advanced therapy platforms, we are required to plan our
production activities and purchase of materials well in
advance. If we suer from third-party raw material short-
ages, underestimate market demand for a product, or
fail to accurately predict when a new product will be
approved for sale, then we may not be able to produce
sucient product to meet demand. These issues could
be made worse during a pandemic, such as the COVID-
19 pandemic, or geopolitical events, such as the war in
Ukraine, and could lead to (i) a sudden increase in
demand for selected medicinal products, resulting in the
short-term unavailability of critical materials; (ii) logisti-
cal and supply challenges that may lead to our inability
to ship products from one place to another due to restric-
tions imposed as a result of a pandemic or geopolitical
events and any related sanctions, which can also impact
transportation and warehousing costs; or (iii) our inabil-
ity to properly operate a manufacturing site due to restric-
tions imposed as the result of a pandemic or any issues
arising from geopolitical events.
Our or our third-party suppliers’ inability to manage
such issues could lead to shutdowns, product shortages,
or to us being entirely unable to supply products to
patients for an extended period of time. Furthermore, as
our products are intended to promote the health of
patients, such shortages or shutdowns could endanger
our reputation and have led to, and could continue to lead
to, significant losses of sales revenue, potential litigation
or allegations that the public health, or the health of indi-
viduals, has been harmed.
Data privacy
Risk description
Noncompliance with personal data protection laws and
regulations
Context and potential impact
We operate in an environment that relies on the collec-
tion, processing, analysis and interpretation of large sets
of patients and other individuals’ personal information,
including via social media and mobile technologies. In
addition, the operation of our business requires data to
flow across the borders of numerous countries in which
there are dierent, potentially conflicting, and frequently
changing, data privacy laws in eect. Examples of such
laws include: the EU General Data Protection Regulation
(GDPR), which took eect in May 2018; the California
Consumer Privacy Act, which took eect in January
2020; Brazil’s General Personal Data Protection Law,
which entered into force in September 2020; and the
Personal Information Protection Law in China, which took
eect in November 2021. Such laws impose stringent
requirements on how we and third parties with whom we
contract collect, share, export or otherwise process per
-
sonal information, and provide for significant penalties
for noncompliance. Breaches of our systems or those of
our third-party contractors, or other failures to protect
the data we collect from misuse or breach by third par-
ties, could expose such personal information to unau-
thorized persons.
Events involving the substantial loss of personal infor-
mation, use of personal information without a legal basis,
or other privacy violations could give rise to significant
liability, reputational harm, damaged relationships with
business partners, and potentially substantial monetary

Item 3. Key Information
17
penalties and other sanctions under laws enacted or
being enacted around the world. Such events could also
lead to restrictions on our ability to use personal infor-
mation and/or transfer personal information across
country borders. In addition, there is a trend of increas-
ing divergence of data privacy legal frameworks, not only
across these frameworks but also within individual legal
frameworks themselves. This divergence may constrain
the implementation of global business processes and
may lead to dierent approaches on the use of health
data for scientific research, which may have a negative
impact on our business and operations.
Falsified medicines
Risk description
Impact of falsified medicines on patient safety, and rep-
utational and financial harm to Novartis and our products
Context and potential impact
We continue to be challenged by the vulnerability of dis-
tribution channels to falsified medicines, which include
counterfeit, stolen, tampered and illegally diverted
medicines as defined by the World Health Organization.
Falsified medicines pose patient safety risks and can
be seriously harmful or life-threatening. Reports of
adverse events related to falsified medicines and
increased levels of falsified medicines in the healthcare
system aect patient confidence in genuine medicines
and in healthcare systems in general. These events could
also cause us substantial reputational and financial harm,
and potentially lead to litigation if the adverse event from
the falsified medicine is mistakenly attributed to the gen-
uine one. Stolen or illegally diverted medicines that are
not properly stored and later sold through unauthorized
channels, could adversely impact patient safety, our rep
-
utation and our business. Furthermore, there is a direct
financial loss when, for example, falsified medicines
replace sales of genuine medicines, or genuine medicines
are recalled following the discovery of falsified products.
Emerging risks
Geopolitical developments
Risk description
Impact of geo- and socio-political threats
Context and potential impact
Challenging political conditions currently exist in various
parts of the world, including an economic downturn; risk
of direct conflicts between nations, such as the war in
Ukraine; a global pandemic; resistance in certain areas
against free trade; anti-corporate sentiment; and social
unrest.
The imposition of taris, including those imposed by
the US and China, and the possibility of additional taris
or other trade restrictions relating to trade could have a
material negative impact on our business. Given that the
outcome of ongoing trade negotiations remains uncer-
tain, we cannot yet determine the nature or extent of the
potential impact on our business. For example, if taris
on pharmaceutical products or active pharmaceutical
ingredients (APIs) were increased, this could impact the
profitability of our products and disrupt our supply chain.
Increasing opposition to free trade may increase the
risks we face in our eorts to improve and harmonize
standards in regulation and intellectual property.
Furthermore, significant conflicts continue in certain
parts of the world. Collectively, such unstable conditions
could, among other things, disturb the international flow
of goods and increase the costs and diculties of inter-
national transactions, which could in turn significantly
impact time to market and our ability to supply our prod-
ucts to patients in an undisrupted fashion, and further
erode reimbursement levels for innovative therapies.
Macroeconomic developments
Risk description
Impact of macroeconomic developments
Context and potential impact
Our business may be impacted by deteriorating macro-
economic and financial conditions directly aecting con-
sumers. Given that patients, in many countries, directly
pay a sizable portion of their own healthcare costs, there
is a risk that consumers may cut back on prescription
drugs due to financial constraints.
Negative macroeconomic developments may also
adversely aect the ability of payers, as well as our dis-
tributors, customers, suppliers, and service providers, to
pay for our products, or otherwise to buy necessary
inventory or raw materials, and to perform their obliga-
tions under agreements with us. Although we make
eorts to monitor the financial condition and liquidity of
these third parties, our ability to do so is limited, and some
of them may become unable to pay their bills in a timely
manner or may even become insolvent. These risks may
be elevated with respect to our interactions with fiscally
challenged government payers, or with third parties with
substantial exposure to such payers.
At the same time, significant changes, and potential
future volatility in financial markets, the consumer and
business environment, the competitive landscape, and
the global political and security landscape make it
increasingly dicult for us to predict our revenues and
earnings. As a result, any revenue or earnings guidance
or outlook that we have given or might give may be over-
taken by events or may otherwise prove to be inaccu-
rate. Although we endeavor to give reasonable estimates
of future revenues and earnings at the time at which we
give such guidance, based on then-current knowledge
and conditions, there is a risk that such guidance or out-
look will prove to be incorrect.
Asset price corrections in financial markets may also
result in lower returns on our financial investments. In
addition, pricing pressures in developed markets result-
ing from eorts to reduce the cost of healthcare (e.g.,
the Inflation Reduction Act in the US, which targets drug
prices) may have a negative impact on our revenue and
our net sales. In addition, inflation has an impact on our
operating costs due to the increased cost of supplies.
Higher costs for energy, raw materials, wages, and cap-
ital will increase our operating costs, potentially reduc-
ing our net sales.

Item 3. Key Information
18
Uncertainties around future central bank and other
economic policies in the US and EU, as well as high debt
levels in some countries could also impact world trade.
Sudden increases in economic, currency or financial
market volatility in dierent countries, such as the recent
appreciation of the US dollar, have also impacted, and
may continue to have an unpredictable impact on our
business, or results of operations, including the conver-
sion of our operating results into our reporting currency,
the US dollar, as well as the value of our investments in
our pension plans.
For a discussion on the eect of price controls on
our business, see “Item 4. Information on the Company—
Item 4.BBusiness overview—Innovative Medicines—
Price controls.” See also “Item 5. Operating and Finan-
cial Review and Prospects—Item 5.B Liquidity and
capital resources—Eects of currency fluctuations,”
“Item 5. Operating and Financial Review and Prospects—
Item 5.B Liquidity and capital resources—Condensed
consolidated balance sheets,” “Item 18. Financial State-
ments—Note 15. Trade receivables” and “Item 18. Finan-
cial Statements—Note 29. Financial instruments – addi-
tional disclosures.”
Climate change
Risk description
Impact of climate change and increased risk of major
natural disasters
Context and potential impact
Novartis is exposed to a broad range of climate risks
such as transition risks (e.g., regulatory frameworks, car-
bon pricing, and the cost of and access to capital) and
physical risks (e.g., heat, water scarcity, sea level rise,
and flooding from severe weather events), which could
vary in magnitude and impact across dierent countries.
Climate change has triggered, and may continue to
trigger, the adoption of new regulatory requirements
across the globe. To comply with such legislation, we
may be required to increase our investment in technol-
ogy to reduce our energy use, water use and greenhouse
gas emissions. In addition, legislative and regulatory
action, both current and in the future, includes or could
include carbon pricing, climate risk related disclosures,
and changes in zoning or building codes to increase cli-
mate resilience. As a result, the combined impact of
these transition risks could increase our direct operat-
ing costs and impact our supply chain. We have also com-
mitted to incorporating the recommendations of the Task
Force on Climate-related Financial Disclosures (TCFD)
framework into our business, which includes providing
qualitative and quantitative disclosures on climate-re-
lated topics on a recurring basis. As a result of these
transition risks, we are committed to becoming carbon
neutral in our own operations by 2025, and carbon neu-
tral across our value chain by 2030. In addition, we are
committed to achieving net zero across our value chain
by 2040. Any failure to achieve these commitments in
the expected time frame, or at all, could result in nega-
tive impacts on our reputation, our operations, and the
price of our shares.
Climate change has created, and will continue to
create, physical risks to our business. Some of our
production facilities that depend on the availability of sig-
nificant water supplies are located in areas where water
is increasingly scarce. Other facilities are located in
areas that, due to increasingly violent weather events,
rising sea levels, or both, are increasingly at risk of sub-
stantial flooding. In regions where such a risk is present,
this has an impact not only on our own operations but
also our distributed supply chain. Such events may result
in the loss of life, increased costs, business interruptions,
destruction of facilities, and disruption to healthcare sys-
tems that patients use to access our medicines.
Furthermore, our corporate headquarters, the head-
quarters of our Innovative Medicines and Sandoz Divi-
sions, and a number of major Innovative Medicines Divi-
sion production and research facilities are located near
earthquake fault lines in Basel, Switzerland. Other major
facilities are located near major earthquake fault lines in
various locations around the world. A major earthquake
could result in loss of life, business interruptions and the
destruction of our facilities.
Tax laws and developments
Risk description
Changes in tax laws or their application
Context and potential impact
Our multinational operations are taxed under the laws
of the countries and other jurisdictions in which we oper-
ate. Changes in tax laws or in their application could lead
to an increased risk of international tax disputes and an
increase in our eective tax rate, which could adversely
aect our financial results. The integrated nature of our
worldwide operations can produce conflicting claims
from revenue authorities in dierent countries as to the
profits to be taxed in the individual countries, including
potential disputes relating to the prices our subsidiaries
charge one another for intercompany transactions,
known as transfer pricing. Most of the jurisdictions in
which we operate have double tax treaties with other
foreign jurisdictions, which provide a framework for mit-
igating the impact of double taxation on our revenues
and capital gains. However, mechanisms developed to
resolve such conflicting claims are largely untried and
can be expected to be very lengthy. Accruals for tax con-
tingencies are made based on experience, interpreta-
tions of tax law, and judgments about potential actions
by tax authorities. However, due to the complexity of tax
contingencies, the ultimate resolution of any tax matter
may result in payments materially dierent from the
amounts accrued.
In 2019, the Organization for Economic Co-operation
and Development (OECD) launched a new initiative on
behalf of the G20 to minimize profit shifting by working
toward a global tax framework that ensures that corpo-
rate income taxes are paid where consumption takes
place, in addition to introducing a global standard on min-
imum taxation combined with new tax dispute resolution
processes. This project achieved OECD political con-
sensus in October 2021, and the detailed principles are
still under discussion by the OECD and political leaders.
The OECD expects that the implementation of these new
principles will begin globally in 2024. Once changes to
the tax laws in any jurisdiction in which the Group

Item 3. Key Information
19
operates are enacted or substantially enacted, the Group
may be subject to the OECD top-up tax, the aim of which
is to bring the total amount of taxes paid on our profit in
a jurisdiction up to a minimum rate of 15%. In 2020, the
EU announced that it would introduce new centralized
taxation powers (which have not yet been introduced) to
address the financial impact of the COVID-19 pandemic.
In addition, the European Commission continues to
extend the application of its policies seeking to limit fis-
cal aid by member states to particular companies,
together with the related investigation into member
states’ practices regarding the issuance of rulings on tax
matters relating to individual companies. Although we
have taken steps to comply with evolving initiatives such
as these of the OECD and the EU, and we will continue
to do so, significant uncertainties remain as to the out-
come of our eorts. For more information, see “Item 18.
Financial Statements—Note 6. Income taxes” and “Item
18. Financial Statements—Note 12. Deferred tax assets
and liabilities.”
General risks
Indebtedness
Risk description
Our indebtedness could adversely aect our operations
Context and potential impact
As of December 31, 2022, we had USD 20.2 billion of
non-current financial debt, and USD 5.9 billion of current
financial debt. Our current and long-term debt requires
us to dedicate a portion of our cash flow to service inter-
est and principal payments and, if interest rates rise, this
amount may increase. As a result, our existing debt may
limit our ability to use our cash flow to fund capital expen-
ditures, to engage in transactions, or to meet other cap-
ital needs, or otherwise may place us at a competitive
disadvantage relative to competitors that have less debt.
Our debt could also limit our flexibility to plan for and
react to changes in our business or industry, and increase
our vulnerability to general adverse economic and indus-
try conditions, including changes in interest rates or a
downturn in our business or the economy. We may also
have diculty refinancing our existing debt or incurring
new debt on terms that we would consider to be com-
mercially reasonable, if at all.
Goodwill and intangible assets
Risk description
Goodwill and intangible assets resulting in significant
impairment charges
Context and potential impact
We carry a significant amount of goodwill and other
intangible assets on our consolidated balance sheet,
including, in particular, substantial goodwill and other
intangible assets obtained through acquisitions, includ-
ing most recently through our acquisitions of Gyroscope
Therapeutics, The Medicines Company, Xiidra, Endo-
cyte, Novartis Gene Therapies, and AAA. As a result, we
may incur significant impairment charges in the future if
the fair value of the intangible assets and the groupings
of cash-generating units containing goodwill would be
less than their carrying value on the Group’s consolidated
balance sheet at any point in time.
We regularly review our intangible and tangible assets
for impairment, including identifiable intangible assets
and goodwill. Any significant impairment charges could
have a material adverse eect on our results of opera-
tions and financial condition. In 2022, for example, we
recorded intangible asset impairment charges of USD
1.3 billion.
For a detailed discussion of how we determine
whether an impairment has occurred, what factors could
result in an impairment, and the impact of impairment
charges on our results of operations, see Item 18. Finan-
cial Statements—Note 1. Significant accounting policies”
and “Item 18. Financial Statements—Note 11. Goodwill
and intangible assets.”
Foreign currency exchange rates
Risk description
Negative eect on financial results due to foreign cur-
rency exchange rate fluctuations
Context and potential impact
Changes in exchange rates between the US dollar, our
reporting currency, and other currencies can result in
significant increases or decreases in our reported sales,
costs and earnings as expressed in US dollars, and in
the reported value of our assets, liabilities and cash flows.
In addition to ordinary market risk, there is a risk that
countries could take armative steps that could signifi-
cantly impact the value of their currencies. Such steps
could include “quantitative easing” measures and poten-
tial withdrawals by countries from common currencies.
In addition, countries facing local financial diculties,
including countries experiencing high inflation rates, and
highly indebted countries facing large capital outflows,
may impose controls on the exchange of foreign cur-
rency. Currency exchange controls and sanctions could
limit our ability to distribute retained earnings from our
local aliates, or to pay intercompany payables due from
those countries.
Despite measures undertaken to reduce or hedge
against foreign currency exchange risks, as a significant
portion of our earnings and expenditures are in curren-
cies other than the US dollar, including expenditures in
Swiss francs that are significantly higher than our reve-
nue in Swiss francs, any such exchange rate volatility
may negatively and materially impact our results of oper-
ations and financial condition, and may impact the
reported value of our net sales, earnings, assets and lia-
bilities. In addition, the timing and extent of such volatil-
ity can be dicult to predict. Furthermore, depending on
the movements of particular foreign exchange rates, we
may be materially adversely aected at a time when the
same currency movements are benefiting some of our
competitors.
For more information on the eects of currency fluc-
tuations on our consolidated financial statements and
on how we manage currency risk, see “Item 5. Operat-
ing and Financial Review and Prospects—Item 5.B Liquid-
ity and capital resources—Effects of currency

Item 3. Key Information
20
fluctuations” and “Item 18. Financial Statements—Note
29. Financial instruments – additional disclosures.”
Key customers
Risk description
Ongoing consolidation among our distributors and retail-
ers, and the concentration of credit risk
Context and potential impact
A significant portion of our global sales is made to a rel-
atively small number of drug wholesalers, retail chains
and other purchasing organizations. For example, our
three most important customers globally accounted for
approximately 16%, 11% and 7%, respectively, of net sales
in 2022. The largest trade receivables outstanding were
for these three customers, amounting to 16%, 14% and
7%, respectively, of the Group’s trade receivables at
December 31, 2022. The trend has been toward further
consolidation among some distributors and retailers. As
a result, we may be aected by fluctuations in the buy-
ing patterns of such customers. Furthermore, these cus-
tomers are gaining additional purchasing leverage,
increasing the pricing pressures facing our businesses.
These pressures can impact our Sandoz Division in par-
ticular, the generic products of which can often be
obtained from numerous competitors. Moreover, we are
exposed to a concentration of credit risk as a result of
this concentration among our customers. If one or more
of our major customers experienced financial diculties,
the eect on us would be substantial, and could include
a substantial loss of sales and an inability to collect
amounts owed to us.
Environmental matters
Risk description
Impact of environmental liabilities
Context and potential impact
The environmental laws of various jurisdictions impose
actual and potential obligations on us to investigate and
remediate contaminated sites, including in connection
with activities in the past by businesses that are no lon-
ger part of Novartis. In some cases, these remediation
eorts may take many years. While we have set aside
provisions for known worldwide environmental liabilities
that are probable and estimable, there is no guarantee
that additional costs will not be incurred beyond the
amounts for which we have provided in the Group
consolidated financial statements. If environmental con-
tamination resulting from our facility operations, busi-
ness activities or products adversely impacts third par-
ties or if we fail to properly manage the safety of our
facilities, including the safety of our employees and con-
tractors, and the environmental risks, we may face sub-
stantial one-time and recurring costs and other penal-
ties, and be required to increase our provisions for
environmental liabilities.
See also “Item 4. Information on the Company—Item
4.D Property, plants and equipment” and “Item 18. Finan-
cial Statements—Note 20. Provisions and other non-cur-
rent liabilities.”
Pension plans
Risk description
Inaccuracies in the assumptions and estimates used to
calculate our pension plan and other post-employment
obligations
Context and potential impact
We sponsor pension and other post-employment bene-
fit plans in various forms that cover a significant portion
of our current and former employees. For post-employ-
ment plans with defined benefit obligations, we are
required to make significant assumptions and estimates
about future events in calculating the expense and the
present value of the liability related to these plans. These
include assumptions about the discount rates we apply
to estimate future defined benefit obligations and net
periodic pension expense, as well as rates of future pen-
sion increases. In addition, our actuarial consultants pro-
vide our management with historical statistical informa-
tion, such as withdrawal and mortality rates in connection
with these estimates.
Assumptions and estimates that we use may dier
materially from the actual results we experience due to
changing market and economic conditions, higher or
lower withdrawal rates, and longer or shorter life spans
of participants, among other factors. Depending on
events, such dierences could have a material eect on
our total equity, and may require us to make additional
contributions to our pension funds.
For more information on obligations under retirement
and other post-employment benefit plans and underly-
ing actuarial assumptions, see “Item 18. Financial State-
ments—Note 25. Post-employment benefits for employ-
ees.”

Item4. Information on the Company
21
Item4. Information on the Company
4.A History and development of Novartis
Novartis AG
Novartis AG was incorporated on February29, 1996,
under the laws of Switzerland as a stock corporation
(“Aktiengesellschaft”) with an indefinite duration. On
December 20, 1996, our predecessor companies,
Ciba-Geigy AG and Sandoz AG, merged into this new
entity, creating Novartis. We are domiciled in and gov-
erned by the laws of Switzerland. Our registered oce
is located at the following address:
Novartis AG
Lichtstrasse 35
CH-4056 Basel, Switzerland
Telephone: +41-61-324-1111
Web: www.novartis.com
Novartis is a multinational group of companies special-
izing in the research, development, manufacturing and
marketing of a broad range of innovative pharmaceuticals
and cost-saving generic medicines. Novartis AG, our
Swiss holding company, owns, directly or indirectly, all
of our significant operating companies. For a list of our
significant operating subsidiaries, see “Item 18. Financial
Statements—Note 31. Principal Group subsidiaries and
associated companies.”
For a description of important corporate developments
since January 1, 2020, see “Item 18. Financial State-
ments—Note 2. Significant transactions.” For information
regarding the Company’s material commitments for cap-
ital expenditures, see “Item 5. Operating and Financial
Review and Prospects—Liquidity and Capital Resources—
Material short- and long-term cash requirements.”
The SEC maintains an internet site at http://www.sec.
gov that contains reports, information statements, and
other information regarding issuers that file electroni-
cally with the SEC.
4.B Business overview
Overview
Our purpose is to reimagine medicine to improve and
extend people’s lives. We use innovative science and
technology to address some of society’s most challeng-
ing healthcare issues. We discover and develop break-
through treatments and find new ways to deliver them to
as many people as possible. We also aim to reward those
who invest their money, time and ideas in our Company.
Our vision is to become the most valued and trusted
medicines company in the world. Our strategy is to
deliver high-value medicines that alleviate society’s
greatest disease burdens through technology leadership
in research and development (R&D) and novel access
approaches. To support this strategy, we have clear
focus areas and priorities, ensuring we deliver on our
purpose and continue to create value for both stakehold-
ers and society. See, “Item 5. Operating and Financial
Review and Prospects—Item 5.A Operating Results—
Overview—Our strategy.”
In 2022, Novartis achieved net sales from continuing
operations of USD 50.5 billion, and total net income
amounted to USD 7.0 billion. Headquartered in Basel,
Switzerland, our Group companies employed approxi-
mately 102 000 full-time equivalent employees as of
December31, 2022. Our products are sold in approxi-
mately 140 countries around the world.
The Group comprises two global operating divisions:
• Innovative Medicines: innovative patent-protected pre-
scription medicines
For a description of our Innovative Medicines Division,
see “—Innovative Medicines—Overview” below.
• Sandoz: generic pharmaceuticals and biosimilars
For a description of our Sandoz Division, see “—
Sandoz” below.
In April 2022, we announced a new, integrated organi-
zational structure and operating model designed to sup-
port our innovation, growth, and productivity ambitions
as a focused medicines company. As part of this new
organizational structure, we have integrated our former
Pharmaceuticals and Oncology business units and cre-
ated two separate commercial organizations—Innovative
Medicines US and Innovative Medicines International.
The Innovative Medicines Division focuses on five core
therapeutic areas—cardiovascular, immunology, neuro-
science, solid tumor, and hematology—as well as other
promoted brands (in the therapeutic areas of ophthal-
mology and respiratory) and established brands. For
more information, see “Item 4. Information on the Com-
pany—Item 4.B Innovative Medicines.” We have also cre-
ated a new Strategy and Growth function that combines
corporate strategy, R&D portfolio strategy and business
development. The purpose of our Strategy and Growth
function is to help drive the company’s growth strategy

Item4. Information on the Company
22
end-to-end and look across internal and external oppor-
tunities to strengthen the Novartis pipeline with medicines
that are both transformational and can make significant
contributions to growth. Finally, we have combined our
former Novartis Technical Operations and Customer &
Technology Solutions units to create a new operations
unit called Operations. This new unit seeks to provide a
stronger and simpler operational backbone that can
accelerate multiple technology transformation initiatives
more eciently, create novel digital solutions at scale,
and increase productivity, while maintaining indus-
try-leading quality and service levels.
Under this new organizational structure, our divisions
are supported by the following organizational units: the
Novartis Institutes for BioMedical Research (NIBR),
Global Drug Development (GDD), and Operations. The
financial results of these organizational units are included
in the results of the divisions for which their work is per-
formed. For more information about NIBR, see “—
Innovative Medicines—Research and development—
Research program” below. For more information about
GDD, see “—Innovative Medicines—Research and devel-
opment—Development program” below. For more infor-
mation about Operations, see “—Item 4.D Property,
plants and equipment” and “Item 18. Financial State
-
ments—Note 3. Segmentation of key figures 2022, 2021
and 2020.”
Corporate activities
We separately report the results of Corporate activities.
The financial results of our Corporate activities include
the costs of the Group headquarters and those of cor-
porate coordination functions in major countries. In addi-
tion, Corporate includes other items of income and
expense that are not attributable to specific segments,
such as certain revenues from intellectual property
rights and certain expenses related to post-employment
benefits, environmental remediation liabilities, charita-
ble activities, donations and sponsorships.
Innovative Medicines
Overview
Our Innovative Medicines Division is a world leader in
oering patent-protected medicines to patients and phy-
sicians. The Innovative Medicines Division researches,
develops, manufactures, distributes and sells patented
pharmaceuticals. The Innovative Medicines Division is
organized into two commercial organizational units—
Innovative Medicines US and Innovative Medicines Inter-
national. These units were created in April 2022 as part
of our new, integrated organizational structure. Prior to
April 2022, the Innovative Medicines Division was orga-
nized into two global business units: Novartis Oncology
and Novartis Pharmaceuticals. See “Item 4. Information
on the Company—Item 4.B Overview.”
The Innovative Medicines Division focuses on core
therapeutic areas—cardiovascular, immunology, neuro-
science, solid tumor, and hematology—as well as other
promoted brands (in the therapeutic areas of ophthal-
mology and respiratory) and established brands.
The Innovative Medicines Division is the larger of our
two divisions in terms of consolidated net sales. It
reported consolidated net sales of USD 41.3 billion in
2022, which represented 81.7% of the Group’s net sales.
The product portfolio of the Innovative Medicines Division
includes a significant number of key marketed products,
many of which are among the leaders in their respective
therapeutic areas.
Innovative Medicines Division
products
The following summaries describe certain key marketed
products in our Innovative Medicines Division, listed
according to year-end net sales within each therapeutic
area or reporting category. Some of the products
described below have lost patent protection or are oth-
erwise subject to generic competition. Others are sub-
ject to patent challenges by potential generic competi-
tors. Please see “—Intellectual property” for general
information on intellectual property and regulatory data
protection, and for more information on the status of pat-
ents and exclusivity for Innovative Medicines Division
products.
While we typically seek to sell our marketed products
throughout the world, not all products and indications
are available in every country. The indications described
in these summaries may therefore vary by country. In
addition, a product may be available under dierent
brand names depending on country and indication.
Key marketed products
Cardiovascular
• Entresto (sacubitril/valsartan) is an oral, first-in-class
angiotensin receptor neprilysin inhibitor. Entresto
enhances the protective eects of a hormone system
called the natriuretic peptide system, and simultane-
ously suppresses the harmful eects of a hormone sys-
tem called the renin-angiotensin-aldosterone system.
It is approved:
• In the US, the EU and other countries to treat adults
who have symptomatic heart failure with reduced
ejection fraction (HFrEF). HFrEF is a disease in which
the heart cannot pump enough blood.
• In the US and other countries to treat most heart fail-
ure patients with preserved ejection fraction (HFpEF).
HFpEF is another disease in which the heart cannot
pump enough blood.
• In the US and other countries to treat children aged
1 year and older who have symptomatic heart failure
with systemic left ventricular systolic dysfunction

Item4. Information on the Company
23
• In China and Japan to treat patients with essential
hypertension (a type of high blood pressure)
• Leqvio (inclisiran) is the first and only small-interfering
RNA therapy to reduce LDL cholesterol, a risk factor
for atherosclerotic cardiovascular disease (ASCVD),
which is caused by plaque buildup in the arteries.
Leqvio is administered by a healthcare professional
twice a year as an injection, following an initial dose
and a dose at three months. It is approved:
• In the EU and other countries to treat adults with pri-
mary hypercholesterolemia (heterozygous familial
and non-familial) or mixed dyslipidemia. In patients
unable to reach LDL cholesterol goals, Leqvio is used
in combination with the maximum tolerated dose of
a statin, or alone or in combination with other lip-
id-lowering therapies in patients who are statin-in-
tolerant or for whom a statin is contraindicated. Pri-
mary hypercholesterolemia and mixed dyslipidemia
are disorders characterized by high levels of fats in
the blood.
• In the US to treat adults with clinical ASCVD or het-
erozygous familial hypercholesterolemia (HeFH), as
an adjunct to diet and maximally tolerated statin ther-
apy, who require additional lowering of LDL choles-
terol. HeFH is an inherited disorder that causes dan-
gerously high levels of LDL cholesterol. (The eect
of Leqvio on cardiovascular morbidity and mortality
has not yet been determined).
Novartis obtained global rights to develop, manufac-
ture and commercialize Leqvio under a license and col-
laboration agreement with Alnylam Pharma ceuticals,
Inc.
Immunology
• Cosentyx (secukinumab) is an injectable, fully human
monoclonal antibody that selectively inhibits interleu-
kin-17A (IL-17A), a cytokine involved in several immuno-
logical diseases. It is approved in the US, the EU and
other countries to treat:
• Adults and children aged 6 years and older with mod-
erate-to-severe plaque psoriasis. Psoriasis is a debil-
itating systemic inflammatory disease that is charac
-
terized by the appearance of raised, red patches on
the skin.
• Adults with active ankylosing spondylitis (AS). AS is
a progressive inflammatory disease that is charac-
terized by chronic back pain, is generally visible on
X-rays, and can cause structural damage to the
bones and joints.
• Adults with active non-radiographic axial spondy-
loarthritis (nr-axSpA). This is a long-term inflamma-
tory disease that is characterized by chronic back
pain and is not visible on X-rays.
• Adults and children (aged 2 years and older in the
US and 6 years and older in the EU) with active pso-
riatic arthritis (PsA). PsA is a type of progressive
inflammatory arthritis that results in swollen and pain-
ful joints and tendons, which can cause structural
damage to the bones and joints.
• Children (aged 4 years and older in the US and 6
years and older in the EU) with enthesitis-related
arthritis (ERA) and children (aged 2 years and older
in the US and 6 years and older in the EU) with juve-
nile psoriatic arthritis (JPsA). ERA and JPsA are sub-
types of juvenile idiopathic arthritis. If left untreated,
they can lead to high levels of pain and disability.
• Xolair (omalizumab) is an injectable prescription med-
icine and the only approved antibody designed to tar-
get and block immunoglobulin E (IgE). It is approved in
the US, the EU and other countries to treat:
• Adults and children aged 6 years and older with mod-
erate-to-severe, or severe, persistent allergic asthma
• Adults and children aged 12 years and older with
chronic spontaneous urticaria/chronic idiopathic
urticaria (hives)
• Adults with nasal polyps or severe chronic rhinosi-
nusitis with nasal polyps (CRSwNP). CRSwNP is a
chronic inflammation of the nose and the sinuses
with the presence of benign lesions (nasal polyps)
on the lining of the nasal sinuses or nasal cavity.
Approved indications vary by country. Xolair is provided
as lyophilized powder for reconstitution, and as liquid
formulation in a pre-filled syringe. Novartis co-pro-
motes Xolair with Genentech in the US and shares a
portion of operating income, but Novartis does not
record any US sales. Novartis records all sales of Xolair
outside the US. For more information, see “Item 18.
Financial Statements—Note 27. Transactions with
related parties—Roche Holding AG.”
• Ilaris (canakinumab) is an injectable, selective, high-af-
finity, fully human monoclonal antibody that inhibits
interleukin-1 beta (IL-1 beta), a key cytokine in the
inflammatory pathway. It is approved in the US, the EU
and other countries to treat patients with certain debil-
itating autoinflammatory disorders, including:
• Adults and children with periodic fever syndromes.
Periodic fever syndromes are a set of rare disorders
characterized by recurrent episodes of illness, with
fever as the main symptom.
• Patients with Still’s disease, including systemic juve-
nile idiopathic arthritis and adult-onset Still’s disease.
Still’s disease is a disorder that causes fevers, rash
and joint pain.
• Adults with acute gouty arthritis. Gouty arthritis is a
type of arthritis characterized by pain, redness, ten-
derness and swelling in one or more joints.
Approved indications vary by country.
Neuroscience
• Gilenya (fingolimod) is an oral sphingosine-1-phos-
phate (S1P) receptor modulator that inhibits the move-
ment of lymphocytes (a type of white blood cell) out of
the lymph nodes into the central nervous system,
thereby preventing nerve inflammation and nervous tis-
sue damage. It is approved:
• In the US to treat adults and children aged 10 years
and older with relapsing forms of multiple sclerosis,
including clinically isolated syndrome, relapsing-re-
mitting multiple sclerosis (RRMS) and active second-
ary progressive multiple sclerosis (SPMS). Multiple

Item4. Information on the Company
24
sclerosis is a disease in which the immune system
attacks the protective covering of nerves (known as
myelin).
• In the EU to treat adults and children aged 10 years
and older who have highly active RRMS despite treat-
ment with at least one disease-modifying agent, or
who have rapidly evolving severe RRMS
Gilenya is licensed from Mitsubishi Tanabe Pharma
Corporation.
• Zolgensma (onasemnogene abeparvovec) is a one-
time intravenous gene therapy designed to address the
genetic root cause of spinal muscular atrophy (SMA)
by replacing the function of the missing or nonworking
SMN1 gene. Zolgensma delivers a new working copy
of the SMN1 gene into a patient’s cells. It is approved
in the US, the EU and other countries to treat:
• Babies and young children who have SMA with bial-
lelic mutations in the SMN1 gene. SMA is a rare,
genetic neuromuscular disease resulting in the pro-
gressive and irreversible loss of motor neurons,
which causes muscle weakness and atrophy.
• Kesimpta (ofatumumab) is an anti-CD20 monoclonal
antibody that enables the targeted depletion of B-cells,
specifically in lymph nodes. Kesimpta is self-adminis-
tered as a once-monthly injection via the Sensoready
autoinjector pen. It is approved:
• In the US to treat adults with relapsing forms of mul-
tiple sclerosis, including clinically isolated syndrome,
relapsing-remitting multiple sclerosis (RRMS) and
active secondary progressive multiple sclerosis
(SPMS). Multiple sclerosis is a disease in which the
immune system attacks the protective covering of
nerves (known as myelin).
• In the EU to treat adults with relapsing forms of mul-
tiple sclerosis with active disease defined by clinical
or imaging features (i.e., relapse, disability, or lesions
detected by MRI scans)
Approved indications vary across other countries. Ofa-
tumumab was originally developed by Genmab and
licensed to GlaxoSmithKline (GSK). Novartis obtained
the rights to ofatumumab from GSK across all indica-
tions.
Solid Tumor
• Tafinlar + Mekinist (dabrafenib + trametinib) is an oral
combination therapy. Tafinlar and Mekinist are kinase
inhibitors of the BRAF and MEK1/2 proteins, respec-
tively, approved in combination in the US, the EU and
other countries to treat patients who have certain types
of cancer with a change in the BRAF gene (called a
BRAF V600 mutation), including:
• Adults with unresectable or metastatic melanoma
with a BRAF V600 mutation. Melanoma is a form of
skin cancer; unresectable melanoma cannot be
removed with surgery and metastatic melanoma has
spread to other parts of the body. Tafinlar and
Mekinist are also approved as single agents for this
indication.
• Adults with stage III melanoma with a BRAF V600
mutation as an adjuvant treatment (following surgery)
• Adults with advanced non-small cell lung cancer
(NSCLC) with a BRAF V600 mutation. NSCLC is the
most common type of lung cancer.
• Adults with locally advanced or metastatic anaplas-
tic thyroid cancer (ATC) with a BRAF V600 mutation
whose cancer has progressed following treatment,
and who have no satisfactory alternative treatment
options (US). ATC is a rare and aggressive form of
thyroid cancer.
Approved indications vary by country. Novartis has
worldwide exclusive rights to develop, manufacture and
commercialize trametinib granted by Japan Tobacco
Inc.
• Kisqali (ribociclib) is a selective oral cyclin-dependent
inhibitor of kinases 4 and 6 (CDK4/6) with somewhat
greater inhibitory activity against CDK4 vs CDK6 – the
two enzymes involved in the control of cell cycle pro-
gression. Kisqali is approved in the US, the EU and other
countries to treat:
• Pre-, peri- and postmenopausal women, and men
(US), with hormone receptor-positive (HR+)/human
epidermal growth factor receptor 2-negative (HER2-)
locally advanced or metastatic breast cancer, in com-
bination with an aromatase inhibitor as initial endo-
crine-based therapy. HR+/HER2- breast cancer is
the most common subtype of breast cancer.
• Pre-, peri- (EU) and postmenopausal women, and
men (US), with HR+/HER2- locally advanced or met-
astatic breast cancer, in combination with fulvestrant,
as first- or second-line therapy
Kisqali was developed by the Novartis Institutes for
BioMedical Research under a research collaboration
with Astex Pharmaceuticals.
• Piqray (alpelisib) is an oral kinase inhibitor that specif-
ically targets the PIK3CA gene. This is the most com-
monly mutated gene in HR+/HER2- breast cancer, the
most common subtype of breast cancer. Piqray is
approved in the US, the EU and other countries to treat:
• Postmenopausal women, and men, with hormone
receptor-positive (HR+)/human epidermal growth
factor receptor 2-negative (HER2-) locally advanced
or metastatic breast cancer with a PIK3CA mutation.
It is used in combination with fulvestrant after dis-
ease progression while on or following an endo-
crine-based regimen (US), or after disease progres-
sion following endocrine therapy as monotherapy
(EU).
• Pluvicto (lutetium (
177
Lu) vipivotide tetraxetan) is an
intravenous radioligand therapy combining a targeting
compound (a ligand) with a therapeutic radionuclide (a
radioactive particle, in this case lutetium-177). Pluvicto
delivers radiation selectively to PSMA-positive cells
and the surrounding cells. It is approved in the US, the
EU and other countries to treat:
• Adults with a type of advanced cancer that has
spread to other parts of the body (metastatic) called
prostate-specific membrane antigen–positive

Item4. Information on the Company
25
metastatic castration-resistant prostate cancer
(PSMA-positive mCRPC) who have already been
treated with other anticancer treatments (androgen
receptor pathway inhibition and taxane-based che-
motherapy)
Hematology
• Promacta/Revolade (eltrombopag) is a once-daily oral
thrombopoietin receptor agonist that works by stimu-
lating bone marrow cells to produce platelets. It is
approved in the US, the EU and other countries to treat:
• Immune thrombocytopenia (ITP) in patients who have
had an insucient response to or have failed previ-
ous therapies. ITP is a bleeding disorder caused by
an unusually low number of platelets.
• Thrombocytopenia in patients with chronic hepatitis
C to allow them to initiate and maintain interfer-
on-based therapy
• Patients with severe aplastic anemia (SAA). SAA is
a condition in which the body does not produce
enough blood cells
Promacta/Revolade is marketed under a research,
development and license agreement between Novartis
and RPI Finance Trust (dba Royalty Pharma), as
assignee of Ligand Pharmaceuticals.
• Tasigna (nilotinib) is a twice-daily oral tyrosine kinase
inhibitor that acts by blocking the BCRABL protein. It
is approved in the US, the EU and other countries to
treat:
• Patients with Philadelphia chromosome-positive
chronic myeloid leukemia (Ph+ CML) in the chronic
and/or accelerated phase who are resistant or intol-
erant to existing treatment. Ph+ CML is a cancer that
starts in the blood-forming cells of bone marrow.
• Newly diagnosed adults and children with Ph+ CML
in the chronic phase
• Jakavi (ruxolitinib) is an oral inhibitor of the JAK1 and
JAK2 tyrosine kinases. It is the first therapy approved
in the EU and other countries to treat:
• Adults with myelofibrosis (MF), including primary
myelofibrosis, post-polycythemia vera myelofibrosis
and post-essential thrombocythemia myelofibrosis.
MF is a rare blood cancer characterized by abnor-
mal blood cell production and scarring in the bone
marrow, which can lead to an enlarged spleen.
• Adults with polycythemia vera (PV) who are resistant
or intolerant to a medication called hydroxyurea. PV
is a rare blood cancer in which the bone marrow pro-
duces too many red blood cells, resulting in serious
problems like clots.
• Patients aged 12 years and older with acute or chronic
graft-versus-host disease (GvHD) and who have had
an inadequate response to corticosteroids or other
systemic therapies. GvHD occurs in stem-cell trans-
plant patients when donor cells see the recipient’s
healthy cells as foreign and attack them.
Novartis licensed ruxolitinib from Incyte Corporation
for development and commercialization in the
indications of oncology, hematology and graft-versus-
host disease outside the US. Incyte Corporation mar-
kets ruxolitinib as Jakafi
®
in the US.
• Scemblix (asciminib) is an oral kinase inhibitor that
works by binding to the ABL myristoyl pocket. It is
approved:
• In the US, the EU and other countries to treat adults
with Philadelphia chromosome-positive chronic
myeloid leukemia (Ph+ CML) in chronic phase who
have previously been treated with two or more tyro-
sine kinase inhibitors (TKIs). CML is a type of cancer
that starts in the blood-forming cells of the bone mar-
row and invades the blood. There are three phases
of CML: chronic phase, accelerated phase and blast
phase.
• In the US and other countries to treat adults with Ph+
CML in chronic phase with the T315I mutation. Some
patients with CML develop mutations that cause
resistance to TKI therapy, including the T315I muta-
tion, which confers resistance to most available TKIs.
As a result, patients with this mutation have limited
treatment options.
Other Promoted Brands
• Lucentis (ranibizumab) is a humanized, high-anity
antibody fragment that binds to vascular endothelial
growth factor A (VEGFA), a protein that can cause the
growth of blood vessels in the eye, potentially leading
to vision loss. Lucentis is an anti-VEGF therapy that is
injected into the eye. It is approved in the EU and other
countries to treat patients with certain eye conditions,
including:
• Adults with neovascular (wet) age-related macular
degeneration (AMD). Wet AMD develops when
abnormal blood vessels grow under the macula and
leak blood and other fluids in the back of the eye,
which damages the macula.
• Adults with proliferative diabetic retinopathy, moder-
ately severe to severe non-proliferative diabetic ret-
inopathy, and/or diabetic macular edema. These con-
ditions are complications of diabetes.
• Adults with visual impairment due to macular edema
secondary to retinal vein occlusion (branch RVO or
central RVO). Retinal vein occlusionis a blockage of
the branch or central retinal veins, which carry blood
away from the retina.
Approved indications vary by country. Lucentis is
licensed from Genentech, and Novartis holds the rights
to commercialize the product outside the US. Genen-
tech holds the rights to commercialize Lucentis in the
US. For more information, see “Item 18. Financial State-
ments—Note 27. Transactions with related parties—
Roche Holding AG.”
• Xiidra (lifitegrast 0.5%), an LFA-1 antagonist, is a pre-
scription eye drop designed to block the interaction of
two key proteins called ICAM-1 and LFA-1, thereby
reducing inflammation. It is approved in the US and
other countries to treat:
• The signs and symptoms of dry eye disease in adults

Item4. Information on the Company
26
Established Brands
• Sandostatin SC (octreotide acetate for injection) and
Sandostatin LAR (octreotide acetate for injectable sus-
pension) are somatostatin analogs approved in the US,
the EU and other countries to treat:
• Adults with acromegaly that is inadequately con-
trolled by surgery or radiotherapy. Acromegaly is a
chronic disease caused by the oversecretion of
growth hormone.
• Patients with certain symptoms associated with car-
cinoid tumors and other types of functional gastro-
intestinal and pancreatic neuroendocrine tumors
Sandostatin LAR is also approved in the EU and other
countries to treat patients with advanced neuroendo-
crine tumors of the midgut or of unknown primary
tumor origin.
Compounds in development
The following table provides an overview of the key
Innovative Medicines Division projects currently in the
Confirmatory Development stage and may also describe
certain projects in the Exploratory Development stage.
Projects typically enter Confirmatory Development and
become the responsibility of our Global Drug Develop-
ment organization during Phase II testing. (For more
information about our drug development program, see
“—Research and development—Development program.”)
Projects are listed in alphabetical order by compound
code, or by product name where applicable. Projects
include those seeking to develop potential uses of new
molecular entities as well as potential additional indica-
tions or new formulations for already marketed products.
The table below, entitled “Projects removed from the
development table since 2021,” highlights changes to the
table entitled “Selected development projects” from the
previous year.
The year that each project entered the current phase
of development refers to the year of the first patient’s
first visit in the first clinical trial of that phase. For proj-
ects in Phase II, the year refers to the first patient’s first
visit in the first Phase II trial, which can occur before the
Confirmatory Development stage. Prior to 2020, we
reported the current phase based on the year in which
the decision to enter the phase was made. To maintain
continuity, we have included certain previously disclosed
projects, noted below, that have not yet achieved “first
patient, first visit” in any Phase IIII study for the reported
indication and route of administration. We have disclosed
these projects using our previous reporting criteria.
A reference to a project being in registration means
that an application has been submitted to a health author-
ity for marketing approval. Compounds and new indica-
tions in development are subject to required regulatory
approvals and, in certain instances, contractual limita-
tions. These compounds and indications are in various
stages of development throughout the world. It may not
be possible to obtain regulatory approval for any or all
of the new compounds and new indications referred to
in this Form20-F in any country or in every country. See
“—Regulation” for more information on the approval pro-
cess.

Item4. Information on the Company
27
Selected development projects
Year project
entered
Formulation/ current Planned filing
Compound/ Common Mechanism route of development dates/current
product name of action Potential indication Category administration phase phase
AVXS- onasemno- Survival motor neuron Spinal muscular atrophy Neuroscience Intrathecal injection /III
(OAV) gene abepar- (SMN) gene therapy (IT formulation)
vovec
Beovu brolucizumab VEGF inhibitor Diabetic retinopathy Ophthalmology Intravitreal injection /III
CFZ iscalimab CD inhibitor Sjögren’s syndrome Immunology Subcutaneous injection /II
Coartem artemether + PGH- (artemisinin Malaria, Global Health Oral /III
lumefantrine combination therapy) uncomplicated
( kg patients)
Cosentyx secukinumab IL-A inhibitor Hidradenitis suppurativa Immunology Subcutaneous injection US/EU
registration
Giant cell arteritis Immunology Subcutaneous injection /III
Lupus nephritis Immunology Subcutaneous injection /III
Psoriatic arthritis (IV formulation) Immunology Intravenous infusion US registration
Ankylosing spondylitis (IV formulation) Immunology Intravenous infusion US registration
JDQ TBD KRAS inhibitor Non-small cell lung cancer, /L
Solid Tumor Oral /III
KAE cipargamin PfATP inhibitor Malaria, uncomplicated Global Health Oral /II
Malaria, severe Global Health Oral /II
KAF ganaplacide Non-artemisinin Malaria, uncomplicated Global Health Oral /II
plasmodium
falciparum inhibitor
Kisqali ribociclib CDK inhibitor Hormone receptor-positive Solid Tumor Oral /III
(HR+)/human epidermal growth
factor receptor -negative (HER-)
early breast cancer (adjuvant)
Leqvio inclisiran siRNA Secondary prevention of cardiovascular Cardiovascular Subcutaneous injection /III
(regulation of LDLC) events in patients with elevated levels
of LDLC
LNA TBD ANGPTL agonist Knee osteoarthritis Immunology Intra-articular /II
LNP iptacopan CFB inhibitor IgA nephropathy Cardiovascular Oral /III
C glomerulopathy Cardiovascular Oral /III
Paroxysmal nocturnal hemoglobinuria Hematology Oral /III
Atypical hemolytic uremic syndrome Hematology Oral /III
LOU remibrutinib BTK inhibitor Chronic spontaneous urticaria Immunology Oral /III
Sjögren’s syndrome Immunology Oral /II
Multiple sclerosis Neuroscience Oral /III
Lutathera lutetium Radioligand therapy Gastroenteropancreatic Solid Tumor Intravenous infusion /III
Lu targeting SSTR neuroendocrine tumors,
dotatate/
st
line in G/ tumors
lutetium
(
Lu)
oxodotreotide
LXE TBD Proteasome inhibitor Visceral leishmaniasis Global Health Oral /II
MBG sabatolimab TIM- antagonist Myelodysplastic syndrome Hematology Intravenous infusion /III
Unfit acute myeloid leukemia Hematology Intravenous infusion /II
MIJ onfasprodil NRB negative Major depressive disorder Neuroscience Intravenous infusion /II
allosteric modulator
NIS TBD TGF-beta inhibitor Pancreatic cancer,
st
line Solid Tumor Intravenous infusion /III
Piqray alpelisib PIK-alpha inhibitor Ovarian cancer Solid Tumor Oral /III
Pluvicto lutetium Radioligand therapy Metastatic castration-resistant Solid Tumor Intravenous infusion /III
Lu targeting PSMA prostate cancer, pre-taxane
vipivotide
tetraxetan/
lutetium
(
Lu)
vipivotide
tetraxetan
Metastatic hormone-sensitive Solid Tumor Intravenous infusion /III
prostate cancer
1
Project added to selected development projects table in 2022 – entered Confirmatory Development

Item4. Information on the Company
28
Year project
entered
Formulation/ current Planned filing
Compound/ Common Mechanism route of development dates/current
product name of action Potential indication Category administration phase phase
PPY
TBD Gene therapy - Geographic atrophy Ophthalmology Subretinal injection /II
complement
factor I modulation
QGE ligelizumab IgE inhibitor Food allergy Immunology Subcutaneous injection /III
SAF libvatrep TRPV antagonist Chronic ocular surface pain Ophthalmology Topical /II
Scemblix asciminib BCRABL inhibitor Chronic myeloid Hematology Oral /III
leukemia,
st
line
SKO
ensovibep Multispecific DARPin Coronavirus infection Global Health Intravenous infusion Not applicable TBD
/II
(N/A)
TQJ pelacarsen ASO targeting Secondary prevention of cardiovascular Cardiovascular Subcutaneous injection /III
lipoprotein(a) events in patients with elevated levels
of lipoprotein(a)
VAY ianalumab BAFFR inhibitor Autoimmune hepatitis Immunology Subcutaneous injection /II
Lupus nephritis
Immunology Subcutaneous injection /III
Sjögren’s syndrome Immunology Subcutaneous injection /III
Warm autoimmune hemolytic anemia
Hematology Intravenous infusion /III
(wAIHA)
VDT tislelizumab Anti-PD- monoclonal Esophageal cancer,
nd
line Solid Tumor Intravenous infusion N/A US/EU
antibody registration
Non-small cell lung cancer Solid Tumor Intravenous infusion N/A EU registration
Nasopharyngeal carcinoma,
st
line Solid Tumor Intravenous infusion N/A /III
Gastric cancer,
st
line Solid Tumor Intravenous infusion N/A /III
Esophageal cancer,
st
line Solid Tumor Intravenous infusion N/A /III
Localized esophageal cancer Solid Tumor Intravenous infusion N/A /III
Hepatocellular carcinoma,
st
line Solid Tumor Intravenous infusion N/A /III
Small cell lung cancer,
st
line Solid Tumor Intravenous infusion N/A /III
Urothelial cell carcinoma,
st
line
Solid Tumor Intravenous infusion N/A /III
VPM gevokizumab IL- beta antagonist Colorectal cancer,
st
line Solid Tumor Intravenous infusion /I
Xolair omalizumab IgE inhibitor Food allergy Immunology Subcutaneous injection /III
XXB
TBD NPR agonist Hypertension Cardiovascular Subcutaneous injection /II
2
Entered confirmatory development following the acquisition of Gyroscope Thereapeutics.
3
In-licensed from Molecular Partners in 2021 (option deal)
4
No definite submission date can be provided at this time
5
Project added to selected development projects table in 2022 – entered Confirmatory Development
6
Formerly “bladder urothelial cell carcinoma”. Indication language updated in 2022 to reflect latest development plan

Item4. Information on the Company
29
Projects removed from the development table since 2021
Compound/product Potential indication Change Reason
ACZ (canakinumab) Non-small cell lung cancer, adjuvant Removed Development discontinued
Beovu Diabetic macular edema Commercialized
CFZ (iscalimab) Liver transplantation Removed Development discontinued
Cosentyx Ankylosing spondylitis head-to-head study versus Removed Development discontinued
Sandoz biosimilar Hyrimoz (adalimumab)
Cosentyx Lichen Planus Removed Development discontinued
CSJ Asthma Removed Development discontinued
Jakavi Acute graft-versus-host disease Commercialized
Jakavi Chronic graft-versus-host disease Commercialized
Kymriah Relapsed/refractory follicular lymphoma Commercialized
LJN Nonalcoholic steatohepatitis Removed Development discontinued
LMI Huntington’s disease Removed Development discontinued
LNP Membranous nephropathy Removed Development discontinued
Vijoice
PIKCA-related overgrowth spectrum Commercialized
Piqray Triple negative breast cancer Removed Development discontinued
Piqray Human epidermal growth factor Removed Development discontinued
receptor -positive (HER+)
advanced breast cancer
Pluvicto Metastatic castration-resistant prostate cancer, post-taxane Commercialized
QBW (icenticaftor) Chronic obstructive pulmonary disease Removed Development discontinued
QGE (ligelizumab) Chronic spontaneous urticaria Removed Development discontinued
QGE (ligelizumab) Chronic inducible urticaria Removed Development discontinued
Scemblix Chronic myeloid leukemia,
rd
line Commercialized
UNR Presbyopia Removed Development discontinued
1
Formerly listed as BYL719

Item4. Information on the Company
30
Principal markets
The Innovative Medicines Division sells products in approximately 130 countries worldwide. Net sales are primar-
ily concentrated in the US and Europe. The following table sets forth the aggregate 2022 net sales of the Innovative
Medicines Division by region:
Innovative Medicines
net sales
to third parties
USD millions
United States
Europe
Asia, Africa, Australasia
Canada and Latin America
Total
Of which in Established Markets
Of which in Emerging Growth Markets
1
Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand.
Many of our Innovative Medicines Division products are
used for chronic conditions that require patients to con-
sume the product over long periods of time, ranging from
months to years. However, certain of our marketed prod-
ucts and development projects, such as cell and gene
therapies, are administered only once. Net sales of the
vast majority of our products are not subject to material
changes in seasonal demand.
Production
Our primary goal is to ensure the uninterrupted and
timely supply of medicines that meet all product speci-
fications and quality standards, and that are produced
in the most cost-eective and sustainable manner. The
manufacturing of our products is highly regulated by gov-
ernmental health authorities around the world, including
the US Food and Drug Administration (FDA) and Euro-
pean Medicines Agency (EMA). In addition to regulatory
requirements, many of our products involve technically
complex manufacturing processes or require highly spe-
cialized raw materials.
In 2022, we began to integrate Advanced Accelera-
tor Applications (AAA), a Novartis company that focuses
on radioligand therapies, into our existing manufacturing
and supply structure. We manufacture our products
across the following technologies at facilities worldwide:
large molecules, small molecules, cell and gene therapy,
RNA therapy and radioligand therapy (see also “—Item
4.D Property, plants and equipment”). In our manufac-
turing network, we maintain state-of-the-art processes,
with quality as a priority, and require our suppliers to
adhere to the same high standards we expect from our
own people and processes. These processes include:
chemical and biological syntheses; radioisotope han-
dling, which relates to our radioligand therapies; sterile
processing, including CART cell processing; and formu-
lation and packaging. We are constantly working to
improve our existing manufacturing processes, develop
new and innovative technologies, and review and adapt
our manufacturing network to meet our needs and those
of our patients and customers.
We produce raw materials for manufacturing in-house
or purchase them from a number of third-party suppli-
ers. Where possible, we maintain multiple supply sources
so that the business is not dependent on a single or lim-
ited number of suppliers. However, our ability to do so
may at times be limited by regulatory or other require-
ments. We monitor market developments that could have
an adverse eect on the supply of essential materials.
Our suppliers of raw materials are required to comply
with applicable regulations and Novartis quality stan-
dards.
Because the manufacturing of our products is com-
plex and highly regulated by governmental health author-
ities, supply is never guaranteed. If we or our third-party
suppliers fail to comply with applicable regulations, then
there could be a product recall or other disruption to our
production activities. We have experienced supply inter-
ruptions for our products in the past, and there can be
no assurance that supply will not be interrupted again in
the future. However, we have implemented a global man-
ufacturing strategy to maximize business continuity in
case of such events.
Marketing and sales
The Innovative Medicines Division serves customers with
21 564 field force representatives, as of December 31,
2022, including supervisors and administrative person-
nel. These trained representatives present the therapeu-
tic benefits and risks of our products to physicians, phar-
macists, hospitals, insurance groups, managed care
organizations and other healthcare professionals. In the
US, Novartis advertises certain products via digital and
traditional media channels, including the internet, televi-
sion, newspapers and magazines. Novartis also pursues
co-promotion or co-marketing opportunities as well as
licensing and distribution agreements with other com-
panies in various markets.

Item4. Information on the Company
31
The marketplace for healthcare is evolving. Customer
groups beyond prescribers have increasing influence on
treatment decisions and guidelines, while patients con-
tinue to become more informed stakeholders in their
healthcare decisions and look for solutions to meet their
changing needs. Novartis is responding by adapting our
business practices to engage appropriately with patients,
customer groups and other stakeholders, including by
delivering innovative solutions to drive education, access
and improved patient care.
The COVID-19 pandemic has accelerated additional
changes related to marketing and sales techniques in
the healthcare industry. For example, many healthcare
professionals have increased their use of virtual plat-
forms when interacting with pharmaceutical companies,
and prefer to receive information in a more convenient
and personalized way. In response, Novartis is working
to implement a new customer engagement model that
combines traditional face-to-face visits with digital and
other methods of engaging healthcare professionals to
improve the eciency and eectiveness of every inter-
action. We are similarly changing our approach to engag-
ing healthcare systems, payers and other healthcare pro-
viders.
Although specific distribution patterns vary by coun-
try, Novartis generally sells its prescription drugs primar-
ily to wholesale and retail drug distributors, hospitals,
clinics, government agencies and managed healthcare
providers. The growing number of so-called “specialty”
drugs in our portfolio has resulted in increased engage-
ment with specialty pharmacies.
In the US, the US Centers for Medicare & Medicaid
Services (CMS) is the largest single payer for healthcare
services as a result of continuing changes in healthcare
economics and an aging population. In addition, both
commercial and government-sponsored managed care
organizations continue to be among the largest groups
of payers for healthcare services in the US. In other coun-
tries, national health services are often the only signifi-
cant payer for healthcare services. In an eort to control
prescription drug costs, almost all managed care orga-
nizations and national health services use formularies
that list specific drugs that may be reimbursed and/or
the level of reimbursement for each drug. Managed care
organizations and national health services also increas-
ingly use cost-benefit analyses to determine whether or
not newly approved drugs will be added to a formulary
and/or the level of reimbursement for that drug, and to
determine whether or not to continue to reimburse exist-
ing drugs. We have dedicated teams that actively seek
to optimize patient access, including formulary positions,
for our products.
The trend toward consolidation among distributors
and retailers of Innovative Medicines Division products
continues in the US and internationally, both within and
across countries. This has increased our customers’ pur-
chasing leverage and resulted in increased pricing pres-
sure on our products. Moreover, we are exposed to
increased concentration of credit risk as a result of the
consolidation among our customers.
Drug pricing is an increasingly prominent issue in
many countries as healthcare spending continues to rise.
This issue has received significant attention in the US,
especially with the recent passage of the Inflation
Reduction Act (please see “—Price controls” for more
information). At Novartis, we are increasing our eorts
to enable patient access through innovative pricing and
access initiatives in the US, Europe and other markets.
These include contract structures such as pay-over-time
and outcome-based agreements.
In 2021, Novartis reached an agreement with the
National Health Service (NHS) in England to implement
a first-of-its-kind population health management
approach designed to provide faster and broader access
to Leqvio for certain high-risk patients with atheroscle-
rotic cardiovascular disease. Novartis is engaging in sim-
ilar collaborations with other countries.
Additionally, following conditional approval of
Zolgensma in Europe in 2020, Novartis Gene Therapies
established “Day One” early access agreements in mul-
tiple European countries. These agreements support
early patient access by allowing a variety of customiz-
able options, including retroactive rebates, deferred pay-
ments, installment options, outcome-based rebates, and
collaborations with healthcare systems to optimize dis-
ease management. These eorts have expanded glob-
ally, and we now have multiple early access agreements
and pay-for-performance agreements (i.e., out-
come-based arrangements) in place in various markets
around the world. Zolgensma is approved in 45 countries.
Competition
The global pharmaceutical market is highly competitive.
We compete against other major international corpora-
tions that have substantial financial and other resources,
as well as against smaller companies that operate region-
ally or nationally. Competition within the industry is
intense and extends across a wide range of activities,
including pricing, product characteristics, customer ser-
vice, sales and marketing, and research and develop-
ment.
Like other companies selling patented pharma-
ceuticals, Novartis faces challenges from companies
selling competing patented products. Generic forms of
our products may follow the expiry of intellectual prop-
erty protection or regulatory exclusivities, and generic
companies may also gain entry to the market through
successfully challenging our intellectual property rights
and exclusivities. We use appropriate, legally permissi-
ble measures to defend those rights and exclusivities.
(See also “—Intellectual property” below). We also may
face competition from over-the-counter (OTC) products
that do not require a prescription from a physician.
There is ongoing consolidation in the pharmaceuti-
cal industry. At the same time, new entrants are looking
to use their expertise to establish or expand their pres-
ence in healthcare, including technology companies
seeking to benefit from the increasing importance of
data and data management in our industry.
Research and development
The discovery and development of a new drug usually
requires approximately 10 to 15years from the initial
research to bringing a drug to market. This includes

Item4. Information on the Company
32
approximately six to eight years from PhaseI clinical tri-
als to market entry. At each of these steps, there is a
substantial risk that a compound (i.e., drug or biologic)
or other therapeutic candidate will not meet the require-
ments to progress further. In such an event, we may be
required to abandon the development of a potential ther-
apy in which we have made a substantial investment.
We manage our research and development expendi-
tures across our entire portfolio in accordance with our
strategic priorities. We make decisions about whether
or not to proceed with development projects on a proj-
ect-by-project basis. These decisions are based on the
project’s potential to meet a significant unmet medical
need or to improve patient outcomes, the strength of the
science underlying the project, and the potential of the
project (subject to the risks inherent in pharmaceutical
development) to generate significant positive financial
results for the Company. Once a management decision
has been made to proceed with the development of a
particular molecule, the level of research and develop-
ment investment required will be driven by many factors.
These include the medical indications for which it is being
developed, the number of indications being pursued,
whether the molecule is of a chemical or biological
nature, the stage of development, and the level of evi-
dence necessary to demonstrate clinical ecacy and
safety.
Research program
Our research program is conducted by the Novartis Insti-
tutes for BioMedical Research (NIBR), which is the
research and early development innovation engine of
Novartis. NIBR is responsible for the discovery of new
medicines for diseases with unmet medical need. We
focus our work in areas where we believe we can have
the most impact for patients. This requires the hiring and
retention of highly talented employees, a focus on fun-
damental disease mechanisms that are relevant across
dierent disease areas, continuous improvement in tech-
nologies for drug discovery and potential therapies,
working with patients to understand their diseases and
the potential benefits of therapies, close alliances with
clinical and commercial colleagues, and the establish-
ment of strategic external alliances.
Approximately 5 500 full-time-equivalent scientists,
physicians and business professionals work at NIBR
sites in Basel, Switzerland; Cambridge, Massachusetts;
East Hanover, New Jersey; San Diego, California; and
Emeryville, California. They contribute to research into
disease areas such as cardiovascular, renal and meta-
bolic diseases; neuroscience; oncology; hematology;
muscle disorders; ophthalmology; autoimmune diseases;
and respiratory and allergic diseases. Research at the
Friedrich Miescher Institute focuses on basic genetic
and genomic research, and the Novartis Institute for
Tropical Diseases (NITD), in Emeryville, California,
focuses on discovering new medicines to fight tropical
diseases, including malaria and cryptosporidiosis.
All drug candidates go through proof-of-concept tri-
als to enable an early assessment of the safety and e-
cacy of the drug while collecting basic information on
pharmacokinetics and tolerability, and adhering to the
guidance for early clinical testing set forth by health
authorities. Following proof of concept, our Global Drug
Development unit conducts confirmatory trials on the
drug candidates.
In 2022, we integrated the Genomics Institute of the
Novartis Research Foundation (GNF), which is based in
San Diego, US, into NIBR. This enables closer collabo-
ration with colleagues across NIBR and gives greater
access to biological, therapeutic, and translational plat-
forms to researchers across Novartis. The NIBR San
Diego site is focused on developing novel technology to
drive drug discovery research, including regenerative
medicine, small interfering RNA therapy and covalent
drug discovery.
Development program
Our Global Drug Development (GDD) organization
oversees and executes drug development activities,
working collaboratively with NIBR, our commercial orga-
nization and other parts of the Company on our overall
pipeline strategy. The GDD organization includes
centralized global functions such as Regulatory Aairs
and Global Development Operations, and global Devel-
opment Units, and has approximately 12 800 full-time
equivalent employees worldwide.
The traditional model of clinical development consists
of three phases:
PhaseI: The first clinical trials of a new compound –
generally performed in a small number of healthy human
volunteers – to assess the drug’s safety profile, includ-
ing the safe dosage range. These trials also determine
how a drug is absorbed, distributed, metabolized and
excreted, and the duration of its action.
PhaseII: Clinical studies performed with patients who
have the target disease, with the aim of continuing the
PhaseI safety assessment in a larger group, assessing
the ecacy of the drug in the patient population, and
determining the appropriate doses for further evaluation.
PhaseIII: Large-scale clinical studies with several hun-
dred to several thousand patients, which are conducted
to establish the safety and ecacy of the drug in spe-
cific indications for regulatory approval. PhaseIII trials
may also be used to compare a new drug against a cur-
rent standard of care to evaluate the overall benefit-risk
relationship of the new medicine.
In each of these phases, physicians monitor volunteer
patients closely to assess the safety and ecacy of a
potential new drug or indication.
Although we use this traditional model, we have tai-
lored the development process to be simpler, more flex-
ible and more ecient. We divide the development pro-
cess into two stages: Exploratory Development to
establish proof of concept, followed by Confirmatory
Development to confirm the concept in large numbers
of patients. Exploratory Development consists of clini-
cal proof-of-concept (PoC) studies, which are small clin-
ical trials (typically involving between five and 15 patients)
that combine elements of traditional PhaseI/II testing.
NIBR conducts these customized trials, which are
designed to give early insights into issues such as safety,
ecacy and toxicity for a drug in a given indication. Once
a positive proof of concept has been established, the

Item4. Information on the Company
33
drug moves to the Confirmatory Development stage and
becomes the responsibility of GDD. Confirmatory Devel-
opment has elements of traditional PhaseII/III testing
and includes trials aimed at confirming the safety and
ecacy of the drug in the given indication, leading up to
submission of a dossier to health authorities for approval.
This stage can also include trials that compare the drug
to the current standard of care for the disease in order
to evaluate the drug’s overall benefit-risk profile. Further,
with new treatment approaches such as gene therapy
for rare diseases, elements of Exploratory and Confir-
matory Development may be combined and suce for
registration under certain conditions such as high unmet
medical need and clinical data showing highly favorable
benefit-risk. In these cases, additional post-approval
studies may be required by the regulatory authorities to
continue to gather important data to further support
approval.
The vast amount of data that must be collected and
evaluated makes clinical testing the most time-consum-
ing and expensive part of new drug development. The
next stage in the drug development process is to seek
registration for the new drug. For more information, see
“—Regulation.”
Our Innovation Management Board (IMB) is respon-
sible for all strategic aspects of our development port-
folio and oversees our drug development budget as well
as major project phase transitions and milestones fol-
lowing a positive proof-of-concept outcome, including
transitions to Confirmatory Development and the deci-
sion to submit a regulatory application to the health
authorities. The IMB is also responsible for the endorse-
ment of overall development strategy, the endorsement
of development project priorities, and decisions on proj-
ect discontinuations. Our Chief Executive Ocer chairs
the IMB, and other representatives from Novartis senior
management, with expertise spanning multiple fields, are
among its core and extended membership.
Alliances and acquisitions
Our Innovative Medicines Division enters into business
development agreements with other pharmaceutical and
biotechnology companies and with academic and other
institutions to develop new products and access new
markets. We license products that complement our cur-
rent product line and are appropriate to our business
strategy. We focus on strategic alliances and acquisition
activities for key disease areas and indications that we
expect to be growth drivers in the future. We review prod-
ucts and compounds we are considering licensing, using
the same criteria that we use for our own internally dis-
covered drugs.
In February 2022, Novartis completed the acquisition
of Gyroscope Therapeutics Holdings Plc. Through the
acquisition, Novartis added PPY988 (GT005), an inves-
tigational one-time gene therapy for geographic atrophy,
to its portfolio.
For more information about recent business acquisitions,
see “Item 18. Financial Statements—Note 2. Significant
transactions.”
Regulation
The international pharmaceutical industry is highly reg-
ulated. Regulatory authorities around the world admin-
ister numerous laws and regulations regarding the test-
ing, approval, manufacturing, importing, labeling and
marketing of drugs, and review the safety and ecacy
of pharmaceutical products. Extensive controls exist on
the non-clinical and clinical development of pharmaceu-
tical products. These regulatory requirements, and the
implementation of them by local health authorities around
the globe, are a major factor in determining whether a
substance can be developed into a marketable product,
and the amount of time and expense associated with
that development.
Health authorities, including those in the US and the
EU, have high standards of technical evaluation. The
introduction of new pharmaceutical products generally
entails a lengthy approval process. Products must be
authorized or registered prior to marketing, and such
authorization or registration must subsequently be main-
tained. In recent years, the registration process has
required increased testing and documentation for the
approval of new drugs, with a corresponding increase in
the expense of product introduction.
To register a pharmaceutical product, a registration
dossier containing evidence establishing the safety, e-
cacy and quality of the product must be submitted to
regulatory authorities. Generally, a therapeutic product
must be registered in each country in which it will be sold.
In every country, the submission of an application to a
regulatory authority does not guarantee that approval to
market the product will be granted. Although the criteria
for the registration of therapeutic drugs are similar in
most countries, the formal structure of the necessary
registration documents and the specific requirements,
including risk tolerance, of the local health authorities
can vary significantly from country to country. Even if a
drug is registered and marketed in one country, the reg-
istration authority in another country may request addi-
tional information from the pharmaceutical company
prior to registration or even reject the product. A drug
may be approved for dierent indications in dierent
countries.
The registration process generally takes between six
months and several years, depending on the country, the
quality of the data submitted, the eciency of the regis-
tration authority’s procedures, and the nature of the
product. Many countries provide for accelerated pro
-
cessing of registration applications for innovative prod-
ucts of particular therapeutic interest. In recent years,
the US and the EU have made eorts to harmonize reg-
istration requirements in order to achieve shorter devel-
opment and registration times for medical products.
However, the requirement in many countries to negoti-
ate selling prices or reimbursement levels with govern-
ment regulators and other payers can substantially
extend the time until a product may finally be available
to patients.
The following provides a summary of the regulatory
processes in the principal markets served by Innovative
Medicines Division aliates:

Item4. Information on the Company
34
United States
In the US, applications for drug registration are submit-
ted to and reviewed by the FDA. The FDA regulates the
testing, manufacturing, labeling and approval for market-
ing of pharmaceutical products intended for commer-
cialization in the US. The FDA continues to monitor the
safety of pharmaceutical products after they have been
approved for sale in the US market. The pharmaceutical
development and registration process is typically inten-
sive, lengthy and rigorous. When a pharmaceutical com-
pany has gathered data that it believes suciently
demonstrates a drug’s safety, ecacy and quality, the
company may filea New Drug Application (NDA) or Bio-
logics License Application (BLA), as applicable, for the
compound. The NDA or BLA must contain all the scien-
tific information that has been gathered about the com-
pound. This typically includes information regarding the
clinical experiences of patients tested in the drug’s clin-
ical trials. A Supplemental New Drug Application (sNDA)
or Supplemental Biologics License Application (sBLA)
must be filed for new indications and dosage forms for
a previously approved drug.
Once an application is submitted, the FDA assigns
reviewers from its sta, including experts in biopharma-
ceutics, chemistry, clinical microbiology, pharmacology/
toxicology, and statistics. After a complete review, these
content experts provide written evaluations of the NDA
or BLA. These recommendations are consolidated and
are used by senior FDA sta in its final evaluation of the
NDA or BLA. Based on that final evaluation, the FDA then
provides to the NDA or BLA’s sponsor an approval, or a
“complete response” letter if the NDA or BLA applica-
tion is not approved. If not approved, the letter will state
the specific deficiencies in the NDA or BLA that need to
be addressed. The sponsor must then submit an ade-
quate response to the deficiencies in order to restart the
review procedure.
Once the FDA has approved an NDA, BLA, sNDA or
sBLA, the company can make the new drug available for
physicians and other healthcare providers to prescribe.
The drug owner must submit periodic reports to the FDA,
including any cases of adverse reactions. For some med-
ications, the FDA requires additional post-approval stud-
ies (PhaseIV) to evaluate long-term eects or to gather
information on the use of the product under specified
conditions.
Throughout the life cycle of a product, the FDA
requires compliance with standards relating to good lab-
oratory, clinical and manufacturing practices. The FDA
also requires compliance with rules pertaining to the
manner in which we may promote our products.
European Union
In the EU, there are three main procedures for applica-
tion for authorization to market pharmaceutical products
in more than one EU member state at the same time: the
centralized procedure, the mutual recognition procedure
and the decentralized procedure. It is also possible to
obtain a national authorization for products intended for
commercialization in a single EU member state only. The
procedure used for first authorization must continue to
be followed for subsequent changes, e.g., to add an indi-
cation for a licensed product.
Under the centralized procedure, applications are
made to the EMA for an authorization that is valid for the
European Union (all member states). The centralized pro-
cedure is mandatory for all biotechnology products; new
chemical entities in cancer, neurodegenerative disor-
ders, diabetes, AIDS, autoimmune diseases and other
immune dysfunctions; advanced therapy medicines,
such as gene therapy, somatic cell therapy and tis
-
sue-engineered medicines; and orphan medicines
(medicines for rare diseases). It is optional for other new
chemical entities, innovative medicinal products, and
medicines for which authorization would be in the inter-
est of public health. When a pharmaceutical company
has gathered data that it believes suciently demon-
strates a drug’s safety, ecacy and quality, the company
may submit an application to the EMA. The EMA then
receives and validates the application, and the special-
ized committee for human medicines, the CHMP, appoints
a rapporteur and co-rapporteur to review it. They use
experts from their countries to carry out the assessment
but can also draw on expertise from other member states
(“multinational teams”). The entire review cycle must be
completed within 210days, although there are “clock
stops” to allow the company to respond to questions set
forth in the rapporteur and co-rapporteur’s assessment
report and agreed with the CHMP. The first clock stop
is at Day 120 and the clock restarts on Day 121, when the
company’s complete response is received by the EMA.
If there are further aspects of the dossier requiring clar-
ification, the CHMP will issue further questions at Day
180, and may also request an oral explanation, in which
case the sponsor must not only respond to the further
questions but also appear before the committee to jus-
tify its responses. On Day 210, the CHMP will take a vote
to recommend the approval or non-approval of the appli-
cation, and their opinion is transferred to the EC. The
final EC decision under this centralized procedure is a
single decision that is applicable to all member states.
This decision occurs 60days, on average, after a posi-
tive CHMP recommendation.
Under both the mutual recognition procedure (MRP)
and the decentralized procedure (DCP), the assessment
is led by one member state, called the reference mem-
ber state (RMS) which then liaises with other member
states, known as the concerned member states. In the
MRP, the company first obtains a marketing authoriza-
tion in the RMS, which is then recognized by the con-
cerned member states in 90 days. In the DCP, the appli-
cation is done simultaneously in the RMS and all
concerned member states. During the DCP, the RMS
drafts an assessment report within 120days. Within an
additional 90days, the concerned member states review
the application and can issue objections or requests for
additional information. On Day 90, each concerned mem-
ber state must be assured that the product is safe and
eective, and that it will cause no undue risks to the pub-
lic health. Once an agreement has been reached, each
member state grants national marketing authorizations
for the product.
After receiving the marketing authorizations, the
company must submit periodic safety reports to the rel-
evant health authority (EMA for the centralized proce-
dure, national health authorities for DCP or MRP). In addi-
tion, pharmacovigilance measures must be implemented

Item4. Information on the Company
35
and monitored, including the collection, evaluation and
expedited reporting of adverse events, and updates to
risk management plans. For some medications, post-ap-
proval studies (PhaseIV) may be imposed to comple-
ment available data with additional data to evaluate long-
term eects (called a Post-Approval Safety Study, or
PASS) or to gather additional ecacy data (called a
Post-Approval Ecacy Study, or PAES).
European marketing authorizations have an initial
duration of five years. The holder of the marketing autho-
rization must actively apply for its renewal after this first
five-year period. As part of the renewal procedure, the
competent authority performs a full benefit-risk review
of the product. Should the authority conclude that the
benefit-risk balance is no longer positive, the marketing
authorization can be suspended or revoked. Once
renewed, the marketing authorization is valid for an unlim-
ited period, unless it is determined that the product must
be further monitored for safety reasons. In this case, the
authority may require another renewal at 10 years. If the
holder does not apply for renewal, the marketing autho-
rization automatically lapses. Any marketing authoriza-
tion that is not followed within three years of its granting
by the actual placing on the market of the correspond-
ing medicinal product ceases to be valid.
Price controls
In most of the markets where we operate, the prices of
pharmaceutical products are subject to both direct and
indirect price controls and to drug reimbursement pro-
grams with varying price control mechanisms. Due to
increasing political pressure and governmental budget
constraints, we expect these mechanisms to remain
robust – and potentially even strengthened – and to have
a continued negative influence on the prices we are able
to charge for our products.
Direct governmental eorts to control prices
United States: The Inflation Reduction Act of 2022 (the
“Act”) was signed into law, which mandates the negoti-
ation of eligible Medicare Part B and Part D drugs; rede-
signs the Medicare Part D benefit, including a USD 2 000
out-of-pocket cap for Medicare beneficiaries; and
imposes penalties for Medicare drugs that increase in
price faster than the rate of inflation. Under the Act, the
US government is required to negotiate the Medicare
prices of single-sourced small molecule drugs that have
been on the market for seven years following FDA
approval as well as single-sourced biologics that have
been on the market for 11 years after FDA approval.
Medicare drugs with the highest total cost to the US
government will be selected for negotiation once they
become eligible. Exemptions include orphan drugs with
an indication for one rare disease or condition, drugs
with a total cost to the US government of less than
USD200 million, and plasma-derived drugs.
The negotiated price will be publicly available and will
become eective for selected drugs nine years after FDA
approval for eligible small molecules and 13 years after
approval for eligible biologics. The negotiated price will
be implemented as follows:
• 10 eligible Medicare Part D drugs in 2026;
• an additional 15 eligible Medicare Part D drugs in 2027;
• an additional 15 eligible combined Medicare PartB and
Part D drugs in 2028;
• an additional 20 eligible combined Medicare PartB and
Part D drugs in 2029; and
• an additional 20 eligible combined Medicare PartB and
Part D drugs each year after 2029
Novartis will participate in the Medicare negotiation pro-
cess if Novartis drugs are selected. Pharmaceutical man-
ufacturers that choose not to participate in the negotia-
tion process will be subject to an excise tax of up to 95%
of sales. Novartis may also be aected by other provi-
sions of the Act, such as price increase penalties for
Medicare Part D drugs starting in 2022 and for Medicare
Part B drugs in 2023, and rebates on eligible Medicare
Part D sales starting in 2025.
In addition, by December 31, 2022, 20 US states had
passed legislation intended to impact pricing or requir-
ing manufacturer price transparency reporting, with
eight of these states also allowing for drug aordability
(i.e., price control) review boards. The disclosure require-
ments vary by state. Many states require multiple types
of reporting, including for new drug applications, new
drug launches, prior notice of price increases, and quar-
terly or annual reporting. It is expected that state legis-
latures will continue to focus on drug pricing in 2023 and
that similar bills will be passed in more states.
Europe: In Europe, our operations are subject to signif-
icant price and marketing regulations. Many govern-
ments are introducing healthcare reforms in a further
attempt to curb increasing healthcare costs. In some
member states, these include reforms to permit the reim-
bursed use of o-label medicines, despite the presence
of licensed alternatives on the market. In the EU, govern-
ments influence the price of pharmaceutical products
through their control of national healthcare systems that
fund a large part of the cost of such products to patients.
The downward pressure on healthcare costs in general
in the EU, particularly with regard to prescription drugs,
is intense. Increasingly strict analyses are applied when
evaluating the entry of new products, and as a result,
access to innovative medicines is limited based on strict
cost-benefit assessments. In addition, prices for mar-
keted products are referenced within member states and
across international borders, further impacting individ-
ual EU member state pricing. Member states also col-
laborate to enhance pricing transparency and have
started conducting joint health technology assessments,
joint pricing negotiations and/or joint purchasing. As an
additional control for healthcare budgets, some EU coun-
tries have passed legislation to impose further manda-
tory rebates for pharmaceutical products and/or finan-
cial claw-backs on the pharmaceutical industry. The
calculation of these rebates and claw-backs may lack
transparency in some cases and can be dicult to pre-
dict.
Regulations favoring generics and biosimilars
In response to rising healthcare costs, most govern-
ments and private medical care providers have estab-
lished reimbursement schemes that favor the substitu-
tion of generic pharmaceuticals for more expensive

Item4. Information on the Company
36
brand-name pharmaceuticals. All US states have generic
substitution statutes. These statutes permit or require
the dispensing pharmacist to substitute a less expensive
generic drug instead of an original drug. Other countries,
including many European countries, have similar laws.
We expect that the pressure for generic substitution will
continue to increase. In addition, the US, the EU and other
jurisdictions are increasingly introducing laws and regu-
lations encouraging the development of biosimilar ver-
sions of biologic drugs, which can also be expected to
have an impact on pricing.
Cross-border sales
Price controls in one country can have an impact in other
countries as a result of cross-border sales. In the EU,
products that we have sold to customers in countries
with stringent price controls can be legally resold to cus-
tomers in other EU countries at a lower price than the
price at which the product is otherwise available in the
importing country (known as parallel trade). In North
America, products that we have sold to customers in
Canada – which has relatively stringent price controls –
are sometimes resold into the US, again at a lower price
than the price at which the product is otherwise sold in
the US. Such imports from Canada and other countries
into the US are currently illegal in most states. However,
six US states (Colorado, Florida, Minnesota, New Hamp-
shire, New Mexico, and Vermont) have enacted laws
allowing the import of pharmaceutical drugs from select
foreign countries. The Secretary of the US Department
of Health and Human Services (HHS) must certify that
each state’s importation plan is safe and cost-eective
before it can be implemented.
We expect that pressures on pricing will continue
worldwide and will likely increase. Because of these
pressures, there can be no certainty that in every instance
we will be able to charge prices for a product that, in a
particular country or in the aggregate, would enable us
to earn an adequate return on our investment in that
product.
Intellectual property
We attach great importance to intellectual property (IP)
rights – including patents, trademarks, copyrights,
know-how, trade secrets and regulatory data protection
– as essential to our purpose of reimagining medicine to
improve and extend people’s lives, and to protect our
investment in research and development, manufacturing
and marketing. The IP system provides a means to attract
the investments needed to conduct and sustainably
finance innovative R&D, and to manage the risks inher-
ent in our work. For example, we seek IP protection under
applicable laws for significant product developments in
major markets. Among other things, patents may cover
the products themselves, including the product’s active
ingredient or ingredients and its formulation. Patents may
cover processes for manufacturing a product, including
processes for manufacturing intermediate substances
used in the manufacture of the product. Patents may also
cover particular uses of a product, such as its use to treat
a particular disease, or its dosage regimen. In addition,
patents may cover tests for certain diseases or
biomarkers – which can improve patient outcomes when
administered with certain drugs – as well as assays,
research tools and other techniques used to identify new
drugs. The protection aorded, which may vary from
country to country, depends upon the type of patent, its
duration and its scope of coverage.
In the US and other countries, the law recognizes that
product development and review by the FDA and other
health authorities can take an extended period, and pro-
vides an extension of patent term for a period related to
the time taken for the conduct of clinical trials and for
the health authority’s review. However, the length of this
extension and the patents to which it applies cannot be
known in advance and can only be determined after the
product is approved. In practice, it is not uncommon for
patent term extensions (PTEs) or supplementary protec-
tion certificates (SPCs) to not fully account for the time
it took to develop the product and receive marketing
authorization. As a result, it is rarely the case, for exam-
ple, that a `product’s active ingredient(s) will have a full
patent term at the time the product is approved by the
FDA and other health authorities.
In addition to patent protection, various countries pro-
vide regulatory-based protection, including regulatory
data protection (RDP) and/or other market exclusivities,
for a prescribed period of time. RDP is a distinct type of
IP right providing exclusivity that precludes a potential
competitor from filing a regulatory application that relies
on the sponsor’s clinical trial data, or that precludes the
regulatory authority from approving the application for
a set period of time. The RDP period can vary depend-
ing on the type of data included in the sponsor’s appli-
cation. When it is available, market exclusivity, unlike RDP,
may preclude a competitor from obtaining marketing
approval for a product even if a competitor’s application
relies on its own data. RDP and market exclusivity peri-
ods generally run from the date a product is approved,
and so their expiration dates cannot be known with cer-
tainty until the product approval date is known and exclu-
sivity has been granted by the relevant authorities.
United States
Patents
In the US, a patent issued from an application filed today
will receive a term of 20years from the earliest applica-
tion filing date, subject to potential patent term adjust-
ments for delays in patent issuance based upon certain
delays in prosecution by the United States Patent and
Trademark Oce (USPTO). A US pharmaceutical patent
that claims a product, method of treatment using a prod-
uct, or method of manufacturing a product may also be
eligible for a PTE. This type of extension may only extend
the patent term for a maximum of fiveyears, and may not
extend the patent term beyond 14years from regulatory
approval. Only one patent may be extended for a prod-
uct based on FDA review.
RDP and market exclusivity
Separate from patent exclusivities, the FDA may provide
regulatory-based protection, which runs in parallel to any
patent protection.
• A new small-molecule active pharmaceutical ingredi-
ent receives five years of RDP, during which time a com-
petitor generally may not obtain final approval of an

Item4. Information on the Company
37
application to the FDA based on a sponsor’s clinical
data.
• A new biologic active pharmaceutical ingredient
receives 12 years of regulatory-based market exclusiv-
ity, during which time a competitor generally may not
market the same or similar drug.
• The FDA may also request that a sponsor conduct
pediatric studies and, in exchange, it will grant an addi-
tional six-month period of pediatric market exclusivity
if the sponsor makes a timely submission of the reports
of the pediatric studies in response to the FDA’s Writ-
ten Request. The sponsor must also have a pat-
ent-based and/or regulatory-based exclusivity period
for the product to which the pediatric market exclusiv-
ity is appended.
• Orphan drug exclusivity provides seven years of mar-
ket exclusivity for drugs designated by the FDA as
orphan drugs, meaning drugs that treat rare diseases.
During this period, a potential competitor generally may
not market the same or similar drug for the same indi-
cation even if the competitor’s application does not rely
on data from the sponsor.
European Union
Patents
Patent applications in Europe may be filed in the Euro-
pean Patent Oce (EPO) or in a particular country or
countries. The EPO system permits a single application
to be granted for the EU plus other non-EU countries
such as Switzerland, Turkey and the UK. When the EPO
grants a patent, it is then validated in the countries that
the patent owner designates. The term of a patent
granted by the EPO or a European country oce is
20years from the earliest application filing date. Phar-
maceutical patents can be granted a further period of
exclusivity under the SPC system. SPCs are designed,
in part, to account for the time taken to receive market-
ing authorization of a product by the European health
authorities. An SPC may be granted to provide, in com-
bination with the patent, up to 15years of exclusivity from
the date of the first European marketing authorization.
However, an SPC cannot last longer than fiveyears. The
SPC duration may be extended by a further sixmonths
if the product is the subject of an agreed and success-
fully completed pediatric investigation plan. The
post-grant phase of patents, including the SPC system,
is currently administered on a country-by-country basis
under national laws that, while diering, are intended to
(but do not always) have the same eect.
RDP and market exclusivity
Separate from patent exclusivities, the EU provides a
system of regulatory data protection for authorized
human medicines that runs in parallel to any patent pro-
tection. The system for new drugs being approved today
is usually referred to as “8+2+1” because it provides an
initial period of eightyears of data protection, during
which a competitor cannot rely on the relevant data; a
further period of twoyears of market exclusivity, during
which the data can be used to support applications for
marketing authorization but a competitive product can-
not be launched; and a possible one-year extension of
the market exclusivity period if, during the initial eight-year
data exclusivity period, the sponsor registered a new
therapeutic indication with “significant clinical benefit.”
This system generally applies both to national and cen-
tralized authorizations in the EU plus other non-EU coun-
tries such as the UK.
The EU also has an orphan drug exclusivity system
for medicines. If a medicine is designated as an orphan
drug, then it benefits from 10years of market exclusivity
after it is authorized, during which time an application for
the same or similar medicine for the same indication will
not generally be accepted or granted. Under certain cir-
cumstances, this exclusivity can be extended with a
two-year pediatric extension.
Third-party patents and challenges to intellectual
property
Third parties can challenge our IP, including patents, pat-
ent term extensions, RDP and marketing exclusivities
(such as pediatric extensions and orphan drug exclusiv-
ity), through various proceedings. For example, patents
in the US can be challenged in the United States Patent
and Trademark Oce (USPTO) through various pro-
ceedings, including Inter Partes Review (IPR) and Post-
Grant Review (PGR) proceedings. They may also be chal
-
lenged through patent infringement litigation under the
Abbreviated New Drug Application (ANDA) provisions of
the Hatch-Waxman Act or under the Biologics Price
Competition and Innovation Act (BPCIA). In the EU, pat-
ents may be challenged through oppositions in the EPO,
or national patents may be challenged in national courts
or national patent oces. The outcomes of such chal-
lenges can be dicult to predict.
In addition to directly challenging our IP rights, in
some circumstances a competitor may be able to mar-
ket a generic version of one of our products by, for exam-
ple, designing around our patents or marketing the
generic product for non-patent-protected indications, or
filing a separate New Drug Application (NDA) under the
Hatch-Waxman Act (typically referred to as a 505(b)(2)
application). Despite RDP, a competitor could opt to incur
the costs of conducting its own clinical trials and prepar-
ing its own regulatory application, and avoid our RDP
altogether. There is a risk that some countries may seek
to impose limitations on or seek not to recognize the
availability of IP rights for pharmaceutical products, or
limit the extent to which such rights may be enforced.
Also, even though we may own, co-own or in-license pat-
ents protecting our products, and conduct free-
dom-to-operate analyses, a third party may nevertheless
assert that one of our products infringes a third-party
patent for which we do not have a license, seeking rem-
edies such as monetary damages or an injunction against
our continued marketing of the product.
As a result, there can be no assurance that our IP
rights will protect our products or that we will be able to
avoid adverse eects from the loss of IP protection or
from third-party patents in the future.
Intellectual property protection for certain key
marketed products and compounds in development
We present additional details below regarding certain IP
protection for the listed Innovative Medicines Division
products. For each, we identify issued, unexpired

Item4. Information on the Company
38
patents by their general subject matter and, in parenthe-
ses, years of expiry, if relevant, in the US and the EU. The
identified patents are owned, co-owned or exclusively
in-licensed by Novartis and relate to at least one dosage
strength of the product or to the method of treatment or
its use as it is currently approved and marketed or, in the
case of a compound in development, as it is currently
submitted to the FDA and/or the EMA for approval. Iden-
tification of an EU patent refers to national patents in EU
countries and/or to the national patents that have been
derived from a patent granted by the EPO. Novartis may
own, co-own, control or have rights to additional patents,
for example, relating to compound forms, methods of
treatment or use, formulations, devices, processes, prod-
uct-by-process, synthesis, purification and detection.
We identify unexpired RDP periods and, in parenthe-
ses, years of expiry if the relevant marketing authoriza-
tions have been authorized or granted. We identify cer-
tain unexpired patent term extensions and marketing
exclusivities and, in parentheses, years of expiry if they
are granted; their subject matter scope may be limited
and is not specified. Marketing exclusivities and patent
term extensions include orphan drug exclusivity (ODE),
pediatric exclusivity (PE), patent term extension (PTE)
and supplementary protection certificate (SPC). We des-
ignate these as “pending” if they have been applied for
but not granted and include years of expiry if estimable.
Such pending applications ultimately may or may not be
granted.
In the case of the EU, identification of a patent, sup-
plementary protection certificate, marketing exclusivity
or regulatory data protection means grant, authorization
and maintenance in at least one EU country or the UK.
However, it could be pending, not granted, expired or
found invalid in others.
For each product below, we indicate whether there
is current generic or biosimilar competition for one or
more product versions in one or more approved indica-
tions in either the US or one or more EU countries, if IP
is otherwise disclosed. We identify certain enforcement
actions, or ongoing challenges to the disclosed IP, includ-
ing IPRs or PGRs if instituted by the USPTO, that have
not been finally resolved (including appeals) unless
noted. Challenges identified as being in administrative
entities, such as national patent oces, include judicial
appeals from decisions of those entities. Resolution of
challenges to the disclosed IP, which in the EU may
involve IP in one or more EU countries, may include set-
tlement agreements under which Novartis permits or
does not permit future launch of generic versions of our
products before expiration of that IP. We identify certain
material terms of such settlement agreements where
they could have a material adverse eect on our busi-
ness. In other cases, such settlement agreements may
contain confidentiality obligations restricting what may
be disclosed.
In the event that a product listed below does not have
identified patents as described above, we provide infor-
mation only on generic competition.
For additional information regarding commercial arrange-
ments with respect to these products, see “—Key mar-
keted products.”
Cardiovascular
• Entresto. US: Four patents on combination (2023 (4)),
PTE (2025), four PEs (2023, 2023, 2024, 2025); two
patents on complex (2026, 2027), two PEs (2027,
2027); three patents on methods of treatment (2033
(3)); patent on dosage regimen (2036); RDP for new
pediatric patient population (2022), PE (2023); RDP for
labeling changes related to new clinical investigation
(2024). EU: Patent on combination (2023), SPC (2028);
two patents on complex (2026, 2026), two SPCs
(2030, 2030); patent on formulation (2028); patent on
method of use (2034); RDP (2025). There is no generic
competition in the US or the EU. In the US, two combi-
nation patents, the two complex patents, and the dos-
age regimen patent are being challenged in ANDA pro-
ceedings against generic manufacturers. In the EU, one
complex patent and the use patent are being opposed
in the EPO. In some EU countries, the combination pat-
ent or its associated SPC is being challenged by
generic manufacturers.
• Leqvio. US: Two patents on composition of matter
(2027, 2034), PTE pending (2035); two patents on
method of treatment and dosing regimen (2027, 2036);
RDP (2026). EU: One patent on composition of matter
(2033), SPC (2035); RDP (2030). There is no generic
competition in the US or the EU.
Immunology
• Cosentyx. US: Five patents on composition of matter
(2025 (4), 2026), PTE (2029); patent on psoriatic arthri-
tis use (2031); patent on psoriasis use (2032); two pat-
ents on ankylosing spondylitis use (2032, 2033); RDP
(2027). EU: Four patents on composition of matter
(2025 (4)), SPC (2030), PE (2030); patent on psoriasis
use (2031); patent on ankylosing spondylitis use (2031);
RDP (2026). There is no generic competition in the US
or the EU. In the EU, the patent on ankylosing spondy-
litis use is being opposed in the EPO.
• Xolair. US: Two patents on syringe formulation (2024,
2025). EU: Three patents on syringe formulation (2024,
2024, 2025). There is no generic competition in the US
or the EU.
• Ilaris. US: Patent on composition of matter (2024); pat-
ent on cryopyrin-associated periodic syndromes use
(2026); patent on familial Mediterranean fever (FMF)
use (2026); patent on systemic onset juvenile idiopathic
arthritis (SJIA) use (2028); patent on hyperimmuno-
globulin D syndrome and tumor necrosis factor recep-
tor-associated periodic syndrome use (2029); patent
on formulation (2029). EU: Patent on composition of
matter (2021), SPC (2024), PE (2025); patent on SJIA
use (2026); patent on FMF use (2026); two patents on
formulation (2029, 2029). There is no generic compe-
tition in the US or the EU.
Neuroscience
• Gilenya. US: Patent on dosage regimen (2027), PE
(2027); patent on 0.25 mg formulation (2032), PE
(2032); patent on method of treatment (2027). EU: Pat-
ent on formulation (2024), SPC (2026); patent on

Item4. Information on the Company
39
0.25mg formulation (2032); patent on dosing regimen
(2027). There is generic competition in the US and in
most EU countries. In the US, the dosage regimen pat-
ent was challenged in ANDA proceedings against a
generic manufacturer and was found invalid by the US
Court of Appeals for the Federal Circuit in June 2022.
Novartis has filed a petition seeking further review with
the US Supreme Court. Novartis is also enforcing the
method of treatment patent against a generic manu-
facturer. In the EU, Novartis is enforcing the dosing reg-
imen patent against generic manufacturers. The dos-
ing regimen patent is being opposed in the EPO.
• Zolgensma. US: Four patents on composition of mat-
ter (2024, 2024, 2026, 2033), PTE pending (2029);
three patents on methods of treatment (2028, 2028,
2029); ODE for spinal muscular atrophy (SMA) in
patients less than 2 years old with biallelic mutations
in the SMN1 gene (2026); RDP (2031). EU: Three pat-
ents on composition of matter (2024, 2024, 2028), SPC
(2029); two patents on methods of use (2028, 2028),
SPC (2033), SPC pending (2033); ODE for SMA in
patients with a biallelic mutation in the SMN1 gene and
a clinical diagnosis of SMA type 1, or patients with a
biallelic mutation in the SMN1 gene and up to three cop-
ies of the SMN2 gene (2030); RDP (2030). There is no
generic competition in the US or the EU.
• Kesimpta. US: Patent on compound (2031); patent on
dosing regimen (2037). EU: Patent on compound
(2023); patent on use (2023), SPC (2028); patent on
formulation (2028), patent on formulation and use
(2028), SPC (2033); patent on dosing regimen (2037).
There is no generic competition in the US or the EU.
Solid Tumor
• Tafinlar and Mekinist.
Tafinlar. US: Two patents on compound (2030, 2030);
patent on method of treatment (2029). EU: Patent on
compound (2029); RDP (2024). There is no generic
competition in the US or the EU.
Mekinist. US: Patent on compound (2025), PTE (2027);
patent on method of treatment (2025); four patents on
formulation (2032 (4)). EU: Patent on compound (2025),
SPC (2029); patent on formulation (2031); RDP (2025).
There is no generic competition in the US or the EU. In
the EU, the formulation patent is being opposed in the
EPO.
Use of Mekinist with Tafinlar or Tafinlar with Mekinist.
US: Patent on combination (2030); four patents on
method of use of combination (2025, 2030, 2030,
2033); ODE on non-small cell lung cancer (2024); ODE
on adjuvant treatment of melanoma (2025); ODE on
anaplastic thyroid cancer (2025); ODE on metastatic
solid tumors (2025). EU: Patent on combination (2030);
patent on adjuvant for melanoma use (2033). There is
no generic competition in the US or the EU. In the EU,
the adjuvant use patent is being opposed in the EPO.
• Kisqali. US: Three patents on compound (2028, 2030,
2031), PTE (2031); three patents on methods of treat-
ment (2029, 2029, 2031); patent on salt form (2031);
patent for tablet formulation (2036). EU: Patent on com-
pound (2027); patent on compound (2029), SPC
(2032); patent on salt form (2031); patent on methods
of use with letrozole (2034); patent on formulation
(2036); RDP (2027). There is no generic competition
in the US or the EU. In the US, the three compound pat-
ents, the three method of treatment patents, the salt
patent and the formulation patent are being challenged
in ANDA proceedings against generic manufacturers.
In the EU, the method of use patent is being opposed
in the EPO.
• Piqray. US: Patent on compound (2029); patent on com-
pound and use (2029), PTE pending (2033); RDP
(2024). EU: Patent on compound and use (2029), SPC
(2034); RDP (2030). There is no generic competition
in the US or the EU.
• Pluvicto. US: Three patents on composition of matter
(2028, 2028, 2034); RDP (2027). PTE pending. EU:
RDP (2032). There is no generic competition in the US
or the EU.
Hematology
• Promacta/Revolade. US: Patent on compound (2021),
PTE (2022), PE (2023); patent on method of enhanc-
ing platelet production using salt (2023), PE (2023);
patent on salt form and thrombocytopenia use (2025),
PE (2026); five patents on tablet formulations of dier-
ent dose strengths (2027 (5)), five PEs (2028 (5)); ODE
on severe aplastic anemia patients in combination with
standard immunosuppressive therapy (2025). EU: Pat-
ent on compound (2021), SPC (2025), PE (2025); pat-
ent on salt form (2023); patent on severe aplastic ane-
mia use (2028). There is no generic competition in the
US or the EU. In the US, generic manufacturers have
filed ANDAs challenging certain patents other than the
compound patent. In the EU, the severe aplastic ane-
mia use patent is being opposed in the EPO.
• Tasigna. US: Patent on compound (2023), PE (2024);
two patents on salt forms (2026, 2028), two PEs (2027,
2029); patent on polymorph compound form (2026),
PE (2027); two patents on capsule form (2026, 2027),
two PEs (2027, 2028); patent on method of treatment
(2032), PE (2032). EU: Patent on compound (2023);
patent on salt form (2026); patent on polymorph com-
pound form (2026); patent on capsule form (2027); pat-
ent on method of treatment (2030). There is no generic
competition in the US or the EU. In the US, generic man-
ufacturers have filed ANDAs challenging certain pat-
ents other than the compound patent.
• Jakavi. EU: Patent on compound (2026), SPC (2027);
two patents on salt form (2028, 2028); patent on com-
pound for polycythemia vera (PV) use (2026); patent
on salt form for graft-versus-host disease (GvHD) use
(2028). There is no generic competition in the EU.

Item4. Information on the Company
40
• Scemblix. US: Patent on compound (2033), PTE pend-
ing (2035); Patent on polymorph compound form
(2040); RDP (2026); ODE (2028). EU: Patent on com-
pound (2033), SPC pending (2037); RDP (2032);
ODE(2032). There is no generic competition in the US
or the EU.
Other Promoted Brands
• Lucentis. EU: There is generic competition in some EU
markets.
• Xiidra. US: Four patents on compound (2024, 2024,
2025, 2026); two patents on formulation (2024, 2033);
five patents on method of treatment (2024, 2024, 2026,
2029, 2029); one patent on polymorph compound form
(2029). PTE pending. There is no generic competition
in the US. Xiidra is not marketed in the EU. In the US,
the compound, compound and use, formulation,
method of treatment, and polymorph compound form
patents are being challenged in ANDA proceedings
against generic manufacturers.
Established Brands
• Sandostatin SC and Sandostatin LAR:
Sandostatin SC: There is generic competition in the US
and the EU.
Sandostatin LAR: There is generic competition in most
EU countries but no generic competition in the US.
Compounds in development
We provide certain patent information for non-marketed
compounds in development that have been submitted to
the FDA and/or the EMA for registration but have not yet
been approved by either agency. For these products,
Novartis will seek all appropriate RDP, will continue to
seek additional intellectual property protection for sig-
nificant product developments, and will apply for PTEs
and SPCs in keeping with the great importance we attach
to intellectual property.
• VDT482 (tislelizumab). US: Patent on composition of
matter (2033). EU: Patent on composition of matter
(2033).
Sandoz
Our Sandoz Division is a global leader in generic
pharmaceuticals and biosimilars, and sells products in
well over 100 countries. In 2022, the Sandoz Division
achieved consolidated net sales of USD 9.2billion, rep-
resenting 18.3% of the Group’s total net sales. Sandoz
develops, manufactures and markets finished dosage
form medicines as well as intermediary products includ-
ing active pharmaceutical ingredients.
Sandoz is organized globally into three franchises:
Retail Generics, Anti-Infectives and Biopharmaceuticals.
In Retail Generics, Sandoz develops, manufactures and
markets finished dosage forms of small-molecule
pharmaceuticals for sale to third parties across a broad
range of therapeutic areas, including finished dosage
form anti-infectives sold to third parties. In Anti-Infec-
tives, Sandoz manufactures and supplies active pharma-
ceutical ingredients and intermediates – mainly antibiot-
ics – for internal use by Retail Generics and for sale to
third-party customers. In Biopharmaceuticals, Sandoz
develops, manufactures and markets protein- and other
biotechnology-based products, including biosimilars.
The Sandoz strategic ambition is to be the world’s
leading and most valued generics and biosimilars com-
pany . Our divisional strategy focuses on three areas:
developing a broad and consistent pipeline of generic
and biosimilar launches across key geographies and
across a broad range of therapeutic areas; positioning
Sandoz to be “first in” by having a strong pipeline with a
focus on being first to market and “last out” by way of
competitive costs and stable supply; and instilling a true
“generic mindset,” with a focus on priorities, simple and
rapid decision-making, and focused resource allocation.
Sandoz is a global market leader in biosimilars, with
a total of eight approved and marketed products, and a
pipeline of over 15 molecules. In addition to internally
developed projects, our biosimilar portfolio comprises
publicly announced commercialization agreements with
BioCon, Gan & Lee, EirGenix, Polpharma Biologics and
Bio-Thera Solutions Ltd. Availability of our biosimilars
varies by country.
Sandoz is also the global market leader in generic
antibiotics. Its Kundl, Austria, manufacturing site is the
hub of the last fully vertically integrated penicillin pro-
duction chain in Europe, which oers certain competi-
tive advantages including added supply chain resilience.
In January 2020, we closed the previously announced
acquisition of the Japanese business of Aspen Global
Incorporated, consisting of o-patent branded medicines
with a focus on anesthetics and specialty brands.
In July 2020, Sandoz and the Austrian government
announced a planned combined investment of more than
EUR 150 million to enhance the long-term competitive-
ness and supply resilience of European production for
key antibiotics.
In May 2021, Sandoz confirmed details of a previously
announced investment of EUR 100 million in antibiotic
manufacturing technology for its Kundl, Austria, manu-
facturing site, and announced an additional EUR 50 mil-
lion investment in a new sterile production line in Pala-
folls, Spain. In November 2022, Sandoz announced an
additional EUR 50 million investment to support increased
manufacturing capacity for finished dosage form peni-
cillin at its Kundl, Austria, manufacturing site.
In October 2021, Sandoz announced that its planned
acquisition of GSK’s global cephalosporin antibiotics
business, first announced in February 2021, had been
successfully closed.
On October 1, 2021, Sandoz Inc., the US subsidiary
of Sandoz, entered into a settlement agreement with the
Civil Division of the US Department of Justice (DOJ) con-
cerning the department’s years-long pricing investiga-
tion into the US generic drug industry. This settlement

Item4. Information on the Company
41
was an expected outcome of the resolution the company
reached in March 2020 with the DOJ Antitrust Division
regarding the same investigation and underlying con-
duct. As part of the settlement, Sandoz agreed to cer-
tain corporate integrity obligations as part of a corporate
integrity agreement with the Oce of Inspector General
of the US Department of Health and Human Services,
which have now been implemented. The settlement con-
tains no new factual allegations against Sandoz and, in
2020, the Group fully provisioned for this settlement and
disclosed the agreement in principle as part of the March
2020 resolution. For more information, see “Item 18.
Financial Statements—Note 20. Provisions and other
non-current liabilities.”
In August 2022, Novartis announced its intention to
separate the Sandoz business to create a standalone
company by way of a 100% spin-o, concluding the
Strategic Review announced in October 2021. The Stra-
tegic Review determined that a 100% spin-o would be
in the best interests of shareholders as it would create
two standalone companies focused on their respective
growth strategies. The new company is planned to be
incorporated in Switzerland and to be listed on the SIX
Swiss Exchange, with an American Depositary Receipt
(ADR) program in the US. Completion of the transaction
is subject to certain conditions, including consultation
with works councils and employee representatives (as
required), general market conditions, tax rulings and
opinions, final Board of Directors endorsement and
shareholder approval in line with Swiss corporate law.
The transaction is expected to be generally tax neutral
to Novartis, with completion expected in the second half
of 2023.
Key marketed products
The Sandoz global portfolio covers a wide range of therapeutic areas. The following are some of the Sandoz key
marketed products in each of its franchises (availability varies by market):
Retail Generics
Product Originator drug Description
Amoxicillin/clavulanic acid Augmentin
®
Antibiotic
Zoledronic acid Aclasta Osteoporosis treatment
Acetylcysteine Various Mucolytic agent
Tacrolimus Various Immunosuppressive agent
Anti-Infectives
Active ingredients Description
Oral and sterile penicillins Anti-infectives
Oral and sterile cephalosporins Anti-infectives
Clavulanic acid and mixtures with clavulanic acid Beta-lactam inhibitors
Classical and semisynthetic macrolides Anti-infectives
Intermediates Description
Various cephalosporin intermediates Anti-infectives
Macrolide base intermediates Anti-infectives
Various crude compounds produced by fermentation Cyclosporine, ascomycin, rapamycin, mycophenolic acid, etc.
Item4. Information on the Company
42
Biopharmaceuticals
Product Originator drug Description
Omnitrope Genotropin
®
Recombinant human growth hormone to treat growth
disorders and growth hormone deficiency
Binocrit and Epoetin alfa Hexal Eprex
®
/Erypo
®
Recombinant protein (erythropoiesis-stimulating) agent
to treat anemia
Zarzio, Zarxio and Filgrastim Hexal Neupogen
®
Recombinant protein (granulocyte colony-stimulating
factor, short-acting) used in oncology
Glatopa Copaxone
®
Treatment for relapsing forms of multiple sclerosis
Erelzi
Enbrel
®
Fusion protein (TNF-alpha receptor) to treat multiple
immune-mediated inflammatory diseases
Rixathon MabThera
®
Chimeric monoclonal antibody (directed against
CD protein on B-cells) to treat blood cancers
and immunological diseases
Hyrimoz Humira
®
Monoclonal antibody (TNF-alpha antibody) to treat multiple
immune-mediated inflammatory diseases
Zessly Remicade
®
Monoclonal antibody (TNF-alpha antibody) to treat multiple
immune-mediated inflammatory diseases
Ziextenzo Neulasta
®
PEGylated form of a recombinant human granulocyte
colony-stimulating factor (long-acting) to
reduce duration of chemotherapy-induced neutropenia
and incidence of chemotherapy-induced febrile
neutropenia
1
Approved in the US in 2016. In patent litigation with Amgen, which markets Enbrel®, the US District Court of New Jersey ruled against Sandoz in August 2019, which was upheld on
appeal. The decision is final and Sandoz cannot launch its Erelzi product in the US until 2029.
Selected development projects – biosimilars in PhaseIII development and
registration
The following table describes Sandoz biosimilar projects that are in registration trial or in registration with a regu-
latory agency (including filing preparation):
Project/ Common Route of
product name (INN) Mechanism of action Potential indication/indications Therapeutic areas administration Current phase
GP denosumab Anti-RANKL Osteoporosis (same as originator) Endocrinology, Subcutaneous Phase III
monoclonal antibody Neurology
SOK aflibercept Recombinant fusion protein Ophthalmology indication (same as originator) Ophthalmology Intravitreal Phase III
that blocks VEGFA
EGIA
trastuzumab Anti-HER recombinant HER+ cancer tumors Oncology Intravenous Registration
IgG, humanized
monoclonal antibody
DSTA
natalizumab Anti-alpha integrin Multiple sclerosis and Crohn’s disease Neurology, Intravenous Registration
monoclonal antibody Immunology (US only)
HFT, insulin glargine, Long-acting (HFT)/ Diabetes Endocrinology, Subcutaneous Phase III/
SMQ, lispro, aspart rapid-acting insulin Diabetology Phase I
PYB
VVF
bevacizumab Recombinant humanized Solid tumors Oncology Intravenous Registration
monoclonal antibody that
blocks VEGF
1
Development in collaboration with EirGenix, Inc.
2
Development in collaboration with Polpharma Biologics
3
Development in collaboration with Gan & Lee
4
Development in collaboration with Bio-Thera Solutions

Item4. Information on the Company
43
Principal markets
The two largest generics markets in the world – the US and Europe – are the principal markets for Sandoz. The
following table sets forth the aggregate 2022 net sales of Sandoz by region:
Sandoz
net sales
to third parties
USD millions
Europe
United States
Asia, Africa, Australasia
Canada and Latin America
Total
Of which in Established Markets
Of which in Emerging Growth Markets
1
Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand.
Many Sandoz products are used for chronic conditions that require patients to consume the product over long peri-
ods of time, from months to years. Sales of our anti-infective products and over-the-counter cough and cold prod-
ucts are subject to material changes in seasonal demand, while sales of the vast majority of our other products are
not. The COVID-19 pandemic has substantially impacted seasonal variation in recent years.
Production
For information on the production of our products, see
“—Item 4.B Business overview—Innovative Medicines—
Production.”
In September 2020, as part of a broader reorganiza-
tion of Novartis Technical Operations (NTO), we estab-
lished the Sandoz Technical Operations (STO) platform
within NTO. STO focuses on producing generic medicines
for Sandoz, as well as related external supply operations
and supply chain. In October 2021, Sandoz created a
new position, Global Head, Sandoz Operations. This new,
broader role, includes full operational and financial
accountability for manufacturing and supply as of Jan-
uary 1, 2023, and was established in anticipation of the
intended Sandoz 100% spin-o.
Due to impurities found in the active ingredient
batches sourced from third-party manufacturers, we
recalled Sandoz valsartan, losartan and irbesartan prod-
ucts in the second half of 2018 and the first quarter of
2019, and ranitidine film-coated tablets in the second
half of 2019, from several markets, in line with our qual-
ity standards for all of our marketed products. The dis-
covery of nitrosamines in some types of drug products
led several health regulators (e.g., EMA, FDA and others)
to conduct a detailed analysis of these impurities in
aected medicinal products. Novartis works with health
authorities around the world to continuously review all
chemical and biological human medicines for the possi-
ble presence of nitrosamines. The EMA, FDA and other
health authorities have provided guidance to the phar-
maceutical industry to prevent unacceptable levels of
nitrosamines in medicines. The EMA review concluded
in March 2021 for chemical human medicines and in July
2021 for biological human medicines. Based on guidance
from health authorities, any chemical and/or biological
human medicines products identified with a potential risk
for nitrosamines will undergo further testing. For these
products, we have provided initial testing and potential
control strategy updates to the EMA and other health
authorities. Due to constant and rapidly evolving health
authority requirements, the risk assessment and related
testing that we may be required to perform may increase.
We will submit and communicate the final outcome of
any risk assessment and related testing to the relevant
health authorities within their expected time frame, and
make changes to the control strategy update, if neces-
sary.
Beginning in September 2021, we initiated a volun-
tary recall of all finished product batches of losartan and
losartan HCT products exceeding or potentially exceed-
ing acceptable regulatory limits of the losartan azide
impurity in the losartan drug substance. This impurity,
which is viewed as an industrywide issue, was initially
considered a mutagen that may increase the risk of can-
cer over time if allowed to rise above certain levels. This
recall was unrelated to the nitrosamine-related recalls
described above, and supply was re-established in
March 2022. Since the voluntary recall, further informa-
tion has been provided to the EMA by Novartis and other
companies in the industry, and the EMA has concluded
that the losartan azide impurity is to be classified as a
non-mutagenic impurity.
Marketing and sales
Sandoz sells a broad portfolio of products, including the
products of our Retail Generics franchise and biosimi-
lars, to wholesalers, pharmacies, hospitals and other
healthcare outlets. Sandoz adapts its marketing and
sales approach to local decision-making processes,
depending on the structure of the market in each coun-
try.
In response to rising healthcare costs, many govern-
ments and private medical care providers, such as health

Item4. Information on the Company
44
maintenance organizations, have instituted reimburse-
ment schemes that favor the substitution of bioequiva-
lent generic versions of originator pharmaceutical prod-
ucts, such as those sold by our Retail Generics franchise.
In the US, statutes have been enacted by all states that
permit or require pharmacists to substitute a less expen-
sive generic product for the brand-name version of a
drug that has been prescribed to a patient. Generic use
is growing in Europe, but penetration rates in many EU
countries (as a percentage of volume) remain well below
those in the US.
Recent trends have been toward continued consoli-
dation among distributors and retailers of Sandoz prod-
ucts, both in the US and internationally, which has
increased our customers’ purchasing leverage.
Legislative or regulatory changes can have a signifi-
cant impact on our business in a country. For more infor-
mation on such changes, see “—Item 4.B Business over-
view—Innovative Medicines—Price controls.”
Our Anti-Infectives franchise supplies active phar-
maceutical ingredients and intermediates – mainly anti-
biotics – for internal use by Retail Generics and for sale
to the pharmaceutical industry worldwide.
Our Biopharmaceuticals franchise operates in an
already mature market framework in Europe and some
other markets, while the business environment is rapidly
evolving in the US and many international markets. Reg-
ulatory pathways for approving biosimilar products are
at various stages of maturity by market, but in some
cases are still relatively new or still in development. Pol-
icies have not yet been fully defined or implemented
regarding the substitution and reimbursement of biosim-
ilars in many markets, including the US.
Competition
The market for generic products is characterized by
increasing demand for high-quality pharmaceuticals that
can be marketed at lower costs due to comparatively
minimal initial research and development investments.
Increasing pressure on healthcare expenditure and
numerous patent and data exclusivity period expirations
have encouraged more generic product launches, result-
ing in increased competition among the companies sell-
ing generic pharmaceutical products, leading to ongoing
price pressure. In particular, Sandoz faces increased
industrywide pressure on prices for generic products,
particularly in the US, driven by factors including cus-
tomer consolidation and growing competition from other
manufacturers of generic medicines. These factors con-
tributed to a decline in industrywide US sales that began
in 2017 and continued through 2022.
Development and registration
Development of Sandoz Biopharmaceuticals is jointly
overseen by Sandoz and GDD, and is governed by the
IMB. Development and registration activities for Retail
Generics products, and registration activities for Bio-
pharmaceuticals products, are also overseen by Sandoz.
Before a generic pharmaceutical may be marketed,
intensive technical and clinical development work must
be performed to demonstrate, in bioavailability studies,
the bioequivalence of the generic product to the
reference product. Nevertheless, research and develop-
ment costs associated with generic pharmaceuticals are
generally much lower than those of the originator
pharmaceuticals, as no original drug discovery, preclin-
ical studies or clinical trials on dose finding, safety and
ecacy are typically performed by the generics com-
pany. As a result, the dierent focus and lower costs of
the generic pharmaceutical model ultimately allow
generic pharmaceutical products to be oered at lower
prices, which support and contribute to the cost contain-
ment goals of healthcare systems.
While generic pharmaceuticals are follow-on ver-
sions of chemically synthesized molecules, biosimilar
products contain a version of the active substance of an
already approved biological reference medicine. Due to
the inherent variability and complexity of biologic prod-
ucts, including batch-to-batch dierences and variations
following manufacturing changes, the development and
the regulatory pathway of biosimilars dier significantly
from that of generics.
The development of a biosimilar product is much
more technically challenging than the development of a
typical generic small-molecule pharmaceutical. While
generic pharmaceuticals normally do not require clinical
studies in patients, regulators worldwide do still require
such targeted studies for biosimilar products. Interna-
tional regulators are nonetheless increasingly discuss-
ing the potential for “tailored development” (which refers
to proposals that seek to implement a more ecient and
expedited biosimilar development process that elimi-
nates the current need for comparative clinical ecacy
and safety studies of biosimilars, without any resulting
compromise on quality, safety or ecacy) for certain
molecules. Biosimilars are engineered to match the ref-
erence medicine in quality, safety and ecacy. This is
achieved by systematically defining the target range of
the reference medicine and then comparing the biosim-
ilar to the reference medicine at various development
stages to confirm biosimilarity and to establish that there
are no clinically meaningful dierences between the pro-
posed biosimilar and the reference biologic. Because
the purpose of a biosimilar clinical development program
is to confirm biosimilarity and not to establish ecacy
and safety de novo, the clinical studies required are less
than those required for a reference biologic. Therefore,
the cost of development for a biosimilar is usually less
than that of a reference biologic.
The development and registration sta employed by
aliates of the Sandoz Division are based worldwide,
including at facilities in Holzkirchen, Germany; Hyder-
abad, India; Kundl, Austria; Ljubljana, Slovenia; and
Rudolstadt, Germany. In November 2020, Sandoz com-
pleted (i) the previously announced closure of the Holz-
kirchen, Germany, development and registration site,
with the exception of patch development and the proj-
ect management group, and (ii) the closure of the prod-
uct development and registration site as well as the main
-
tenance and development regulatory centers in Unterach,
Austria. We conduct an ongoing review of our global
development and regulatory network to consolidate and
streamline operations and optimize our network struc-
ture to enable Sandoz to compete sustainably in an
increasingly challenging generics environment. In 2021,
Sandoz completed the previously announced closures
of its maintenance regulatory center in Barleben, Ger-
many, its Fougera development center located in

Item4. Information on the Company
45
Melville, New York, as well as its product development
center in Boucherville, Canada.
Regulation
Generics
The Hatch-Waxman Act in the US (and similar legislation
in the EU and in other countries) eliminated the require-
ment that manufacturers of generic pharmaceuticals
repeat the extensive clinical trials required for reference
products, so long as the generic version could be shown
to be therapeutically equivalent to the reference prod-
uct.
In the US, the decision on whether a generic phar-
maceutical is therapeutically equivalent to the original
product is made by the FDA based on an Abbreviated
New Drug Application (ANDA) filed by the generic prod-
uct’s manufacturer. An ANDA is generally permitted to
be filed four years after the initial approval of the refer-
ence product and generally cannot be fully approved by
the FDA until any regulatory exclusivity of the reference
product has expired. The process typically takes nearly
two years from the filing of the ANDA until FDA approval.
However, delays can occur if issues arise, for example,
regarding the interpretation of bioequivalence study
data, labeling requirements for the generic product, or
qualifying the supply of active ingredients. In addition,
the Hatch-Waxman Act requires a generic manufacturer
to certify in certain situations that the generic product
does not infringe on any current applicable patents on
the product held by the holder of the marketing authori-
zation for the reference product, or to certify that such
patents are invalid. This certification often results in a
patent infringement lawsuit being brought against the
generics company. In the event of such a lawsuit, the
Hatch-Waxman Act imposes an automatic 30-month
stay in the approval of the ANDA to allow the parties to
resolve the intellectual property issues. For generic
applicants who are the first to file their ANDA containing
a certification claiming non-infringement or patent inva-
lidity, the Hatch-Waxman Act generally provides those
applicants with 180 days of marketing exclusivity,
enabling such generic applicants to exclusively market
their product alongside the reference product at a cer-
tain point in time, which is generally after any intellectual
property issues have been resolved. However, after such
point in time, the generic applicants must launch their
products within certain time frames or risk losing the
marketing exclusivity that they had gained by being a
first-to-file applicant.
In the EU, decisions on the granting of a marketing
authorization are made either by the European Commis-
sion based on a positive recommendation by the EMA
under the centralized procedure, or by a single member
state under the national or decentralized procedure. See
“—Innovative Medicines—Regulation—European Union.”
Companies may submit abridged applications for
approval of a generic medicinal product based upon its
“essential similarity” to a medicinal product authorized
and marketed in the EU following the expiration of the
product’s data exclusivity period. In such cases, the
generics company is able to submit its abridged appli-
cation based on the data submitted by the innovator
company for the reference product, without the need to
conduct extensive PhaseIII clinical trials of its own. For
all products that received a marketing authorization in
the EU after late 2005, the abridged application can be
submitted throughout the EU. However, the data submit-
ted by the innovator company in support of its applica-
tion for a marketing authorization for the reference prod-
uct is generally protected for 10 years after the first grant
of marketing authorization in all member states, and can
be extended for an additional year if, during the initial
eight-year data exclusivity period, the innovator company
registers a new therapeutic indication with “significant
clinical benefit.” In the case of orphan drugs, it may be
extended with a two-year pediatric extension. See “—
Item 4.B Business overview—Innovative Medicines—
Intellectual property.”
Biosimilars
The regulatory pathways for approval of biosimilar
medicines are still being developed and established in
many countries of the world. A regulatory framework for
the approval of biosimilars has been established in the
EU, Japan, Canada and the US, while the World Health
Organization (WHO) has issued guidance. Sandoz has
successfully registered and launched the first biosimilar
(or biosimilar-type) medicine in Europe, the US, Canada,
Japan, Taiwan, Australia, and many countries in Latin
America and Asia. Sandoz was the first company to
secure approval for and launch a biosimilar under the US
biosimilar pathway that was established as part of the
Biologics Price Competition and Innovation Act (BPCIA).
The approval of biosimilars in Europe follows a process
similar to that followed for small molecules. However,
biosimilars usually have to be approved through the cen-
tralized procedure because they are manufactured using
recombinant DNA technology. As part of the approval
process in the EU, biosimilars have to demonstrate com-
parability to the reference medicine in terms of safety,
ecacy and quality through an extensive comparability
exercise, based on strict guidelines set by the authori-
ties. Regulators will only approve a biosimilar based on
data that allows the regulators to conclude that there are
no clinically meaningful dierences between the refer-
ence medicine and the biosimilar.
In the US, under the BPCIA, a biosimilar must be
highly similar with no clinically meaningful dierences
compared to the reference medicine. Approval of a bio-
similar in the US requires the submission of a BLA to the
FDA, including an assessment of immunogenicity and
pharmacokinetics; an ecacy study; and possibly a phar
-
macodynamics study. The BLA for a biosimilar can be
submitted as soon as four years after the initial approval
of the reference biologic, but can only be approved
12years after the initial approval of the reference bio-
logic.
Intellectual property
We take all reasonable steps to ensure that our products
do not infringe valid intellectual property rights held by
others, including taking steps to proactively challenge
intellectual property rights that we believe should not
have been granted. Nevertheless, competing companies

Item4. Information on the Company
46
commonly assert patent and other intellectual property
rights. As a result, we can become involved in significant
litigation regarding our products. If we are unsuccessful
in defending these suits, we could be subject to injunc-
tions preventing us from selling our products and to
potentially substantial damages.
Wherever possible, our products are protected by
our own patents. Among other things, patents may cover
the products themselves, including the product’s formu-
lation, or the processes for manufacturing a product.
However, there can be no assurance that our intellectual
property will protect our products or that we will be able
to avoid adverse eects from the loss of intellectual prop-
erty protection in the future.
4.C Organizational structure
Organizational structure
See “Item4. Information on the Company—Item4.A History and development of Novartis” and “Item4. Information
on the Company—Item4.B Business overview—Overview.”
Significant subsidiaries
See “Item 18. Financial Statements—Note 31. Principal Group subsidiaries and associated companies.”
4.D Property, plants and equipment
Our principal executive oces are located in Basel, Swit-
zerland. Our divisions operate through a number of al-
iates that have oces, research and development facil-
ities, and production sites throughout the world.
We generally own our facilities or have entered into
long-term lease arrangements for them. Some of our
principal facilities are subject to mortgages and other
security interests granted to secure certain debts.
Novartis Operations manages the production, supply
chains and quality of our Innovative Medicines and
Sandoz Division products through a network of 55
manufacturing sites, as well as through external suppli-
ers, and warehouse and distribution centers. In addition,
Novartis Operations also manages non-production real
estate owned or leased by Novartis around the world.
The following table sets forth our major headquar-
ters and most significant production, research and devel-
opment, and administrative facilities. See also “—Item
4.B Business overview—Innovative Medicines—Produc-
tion” and “—Item 4.B Business overview—Sandoz—Pro-
duction” for a discussion of our manufacturing pro-
cesses.
Major facilities
Size of site
Location (in square meters) Major activity
Basel, Switzerland – St.Johann Global Group headquarters; global Innovative Medicines Division headquarters;
global Sandoz Division headquarters; research and development;
production of drug substances and drug intermediates
Kundl and Schaftenau, Austria Production of biotechnological products, drug products and finished products,
anti-infectives, active drug substances and nucleic acids; product development
East Hanover, New Jersey Innovative Medicines Division US headquarters; research and development
Barleben, Germany Production of broad range of generics finished dosage forms
Cambridge, Massachusetts Research and development
Menges, Slovenia Production of drug substances and drug intermediates
Shanghai, China Research and development
Stein, Switzerland Production of sterile vials, pre-filled syringes and ampoules; inhalation capsules,
tablets and transdermals; active pharmaceutical ingredients; and cell and gene therapies
Holzkirchen, Germany Sandoz Division production of transdermal delivery systems and certain international
and global service functions.
Huningue, France Production of drug substances for clinical and commercial supply
Durham, North Carolina Manufacture, package and release commercial Zolgensma product
and certain clinical development activities
Princeton, New Jersey Sandoz Division US headquarters
Schweizerhalle, Switzerland Manufacture of small-interfering RNA (siRNA) drug substance for Leqvio

Item4. Information on the Company
47
As our product portfolio evolves, Novartis Operations is
adapting our manufacturing capacity and capabilities to
meet our changing needs, shifting from high-volume
products toward lower-volume, customized and person-
alized medicines. As of December 31, 2022, we have
closed, exited or sold 19 manufacturing sites since 2019
and have announced the closure, exit or sale of seven
additional manufacturing sites. We have continued to
invest in new technologies implemented at our sites, such
as the new targeted radioligand therapy production facil-
ity in Indianapolis, Indiana, which is currently under con-
struction (with an expected size of approximately 67
thousand square meters), the FDA-approved Zolgensma
production site in Durham, North Carolina, and the
small-interfering RNA (siRNA) oligonucleotide manufac-
turing facility in Schweizerhalle, Switzerland. We are
leveraging innovation to increase the reliability and pro-
ductivity of our manufacturing network, including using
data and digital technologies. We continue to seek
opportunities to manage our production facilities as
eciently as possible, optimize external spend, and sim-
plify and standardize across our manufacturing network
to help us increase our cost competitiveness and opti-
mize the value of our products. At the same time, we are
working to improve our environmental sustainability, for
example by reducing energy, waste disposal and water
consumption at our sites by making our manufacturing
processes more ecient, introducing new technologies,
and switching to clean and renewable energy solutions.
For a description of the impact of environmental mat-
ters, see “Item3. Key Information—Item3.D Risk fac-
tors—Environmental, social and governance matters—
Failure to meet increasingly challenging environmental,
social and governance expectations,” “Item 3. Key Infor-
mation—Item 3.D Risk factors—Environmental matters—
Impact of environmental liabilities,” and “Item 3. Key Infor-
mation—Item 3.D Risk factors—Climate change—Climate
change and increased risk of major natural disasters.”
See also “Item 18. Financial Statements—Note20. Pro-
visions and other non-current liabilities.”

Item4A. Unresolved Sta Comments
48
Item4A. Unresolved Sta Comments
Not applicable.

Item 5. Operating and Financial Review andProspects
49
Item 5. Operating and Financial Review
andProspects
5.A Operating results
This operating and financial review should be read with
the Group’s consolidated financial statements in this
Annual Report, which have been prepared in accordance
with International Financial Reporting Standards (IFRS)
as published by the International Accounting Standards
Board (see “Item 18. Financial Statements”). “Item 5.
Operating and Financial Review and Prospects” with the
sections on compounds in development and selected
development projects of our divisions (see “Item 4. Infor-
mation on the Company—Item 4.B Business overview”)
constitute the Operating and Financial Review (Lage-
bericht), as defined by the Swiss Code of Obligations.
The discussion and analysis of the financial condition
and results of operations of certain items from fiscal year
ended December 31, 2020, and year-to-year compari-
son between fiscal year ended December 31, 2021, and
December 31, 2020, that are not included in this Form
20-F can be found in “Item 5. Operating and Financial
Review and Prospects” of our Form 20-F for the fiscal
year ended December 31, 2021, which is incorporated
by reference herein.
Overview
Our purpose is to reimagine medicine to improve and
extend people’s lives. We use innovative science and tech-
nology to address some of society’s most challenging
healthcare issues. We discover and develop breakthrough
treatments and find new ways to deliver them to as many
people as possible. We also aim to reward those who invest
their money, time and ideas in our Company. Our vision is
to become the most valued and trusted medicines com-
pany in the world.
The businesses of Novartis are divided operationally
on a worldwide basis into two identified reporting seg-
ments:
• Innovative Medicines: innovative patent-protected pre-
scription medicines
• Sandoz: generic pharmaceuticals and biosimilars
In addition, we separately report the results of Corpo-
rate activities. The financial results of our Corporate
activities include the costs of the Group headquarters
and those of corporate coordination functions in major
countries. Corporate also includes other items of income
and expense that are not attributable to specific seg-
ments, such as certain revenues from intellectual prop-
erty rights and certain expenses related to post-employ-
ment benefits, environmental remediation liabilities,
charitable activities, donations and sponsorships.
In April 2022, we announced a new, integrated orga-
nizational structure and operating model designed to sup-
port our innovation, growth, and productivity ambitions as
a focused medicines company. For information about this
new organizational structure, see “Item 4. Information on
the Company—Item 4.B Overview.” Under this new orga-
nizational structure, our divisions are supported by the
following organizational units: the Novartis Institutes for
BioMedical Research, Global Drug Development, and
Novartis Operations. The financial results of these orga-
nizational units are included in the results of the divisions
for which their work is performed.
Significant transactions are discussed in “Item 18.
Financial Statements—Note 2. Significant transactions,”
and “Item 18. Financial Statements—Note 3. Segmenta-
tion of key figures 2022, 2021 and 2020.”
Our business environment
Medical technology continues to accelerate, with new
advanced treatments emerging to meet the growing
need for high-quality healthcare. At the same time, aging
populations are putting pressure on healthcare
resources, while access to healthcare remains a chal-
lenge around the world. As a result, we see challenges
and opportunities in our business environment: the need
for continuous innovation in healthcare, increasing
access to medicines, the adoption of new working prac-
tices and the growing use of data science and technol-
ogy. The following are some major trends currently shap-
ing our business environment.
• Spending on healthcare continues to grow. The need
for high-quality healthcare is more critical than ever.
Over the next five years, global spending on medicines
is forecast to rise faster than GDP in many developed
countries. The price of medicines remains a key issue
as increased healthcare spending and a more uncer-
tain economic outlook weigh on government budgets.
• Aging populations are fueling a rise in chronic illness.
Aging and lifestyle changes are triggering an increase
in noncommunicable diseases, such as cancer, heart
disease and diabetes, causing millions of preventable
deaths and putting further pressure on healthcare
resources.
• Medical science continues to accelerate. Scientific
innovation is advancing at an unprecedented pace. In
recent years, new types of treatments have been
approved, including RNA therapies, gene and cell ther-
apies, and radioligand therapies, which oer targeted
approaches to treating serious diseases. Because
these medicines are complex, they require focused
investment and expertise to bring them to reality for
patients.
• Access to healthcare remains a formidable challenge.
Worldwide, millions of patients struggle to access the
medicines they need. This may be because of cost,
inequity, or structural issues in healthcare systems.
While access to medicines remains an acute issue in
lower-income countries, it is a problem in developed

Item 5. Operating and Financial Review andProspects
50
countries too, where the COVID-19 pandemic high-
lighted that deep health inequities remain entrenched.
• Patients are moving to the center of healthcare. Patients
are demanding more say over their treatment through
patient representative groups and other means. In
response, healthcare systems and pharmaceutical
companies are adapting, moving toward a more inte-
grated, end-to-end approach, with an increased focus
on patient engagement in drug development and other
areas. At the same time, patients are becoming more
important as data owners – as personal data allows
more targeted treatments and supports development
of new medicines.
• Economic uncertainty is growing, post-COVID-19 pan-
demic. The global economy is facing considerable
uncertainty, driven by concerns over rising energy
prices and geopolitical instability. Forecasts suggest
the current economic slowdown is likely to continue in
2023. In our own industry, the COVID-19 pandemic put
strain on supply chains and highlighted the importance
of resilient supplies of active pharmaceutical ingredi-
ents – the raw materials used to make finished
medicines. See “Item 3. Key Information—Item 3.D Risk
factors—Pricing, reimbursement and access—Pricing
and reimbursement pressure, including pricing trans-
parency and access to healthcare,” and “Item 3. Key
Information—Item 3.D Risk factors—Macroeconomic
developments—Impact of macroeconomic develop-
ments.”
• Biopharma searches for more eciency. At a time of
growing economic uncertainty, investors are looking
for sustainable growth in margins and earnings. To
remain competitive, pharmaceutical companies are
moving to more agile, cost-ecient business models,
particularly as they invest to build specialized capabil-
ities in research and development (R&D) and manufac-
turing. Meanwhile, rates of return on R&D are increas-
ing for the first time in several years, largely because
of emergency approvals during the COVID-19 pan-
demic and faster innovation cycles.
• New technologies are reshaping our industry. The use
of data science and technology is increasing across
the industry in everything from R&D to manufacturing
and marketing. This has brought greater eciency, but
it also requires new investment and skills. Importantly,
new technologies are helping close gaps between
companies, healthcare systems and patients – for
example, by providing insights into the social determi-
nants of heart health enabling the development of new
prevention measures.
• Working practices are changing. Working practices are
changing in many countries. Demand for new skills is
increasing, especially in areas such as data science.
Workforces are becoming more flexible and more
diverse, allowing companies to tap into new talent
pools – important at a time of skills shortages in many
parts of the economy.
• Climate change is increasingly aecting human health.
Climate change could undermine decades of progress
in improving human health at a time when antimicrobial
resistance is also rising. At the same time, more gov-
ernments are looking to decarbonize their economies
over the long-term, while companies also face increased
scrutiny over the sustainability of their operations and
supply chains. See “Item 3. Key Information—Item 3.D
Risk factors—Climate change—Impact of climate
change and increased risk of major natural disasters.”
Our strategy
Our strategy as a focused medicines company is to
deliver high-value medicines that alleviate society’s
greatest disease burdens through technology leadership
in R&D and novel access approaches.
We have made significant progress in transforming
Novartis from a diversified healthcare conglomerate into
a focused medicines company. In doing so, we have
divested or spun o non-core businesses and made tar-
geted acquisitions to focus on our core business: dis-
covering and developing new medicines and finding new
ways to deliver them to as many people as possible.
In 2022, we continued to execute on our strategy by
putting in place a new organizational structure to sup-
port innovation, growth and productivity. We also updated
our strategic priorities and announced our intention to
spin-o our Sandoz business, which paves the way for
Novartis to advance as a company focused fully on
innovative medicines. See “Item 4. Information on the
Company—Item 4.B Overview” and “Item 4. Information
on the Company—Item 4.B Sandoz.”
Our strategy has clear focus areas and priorities to
meet the challenges and opportunities we see in our
business environment, and ensure we continue to cre-
ate value for our stakeholders and society.
Our focus areas determine where we invest most of our
time, energy and resources and include:
• Core therapeutic areas with high unmet patient needs:
cardiovascular; immunology; neuroscience; solid
tumors; and hematology.
• Technology platforms where we have the depth and
scale to discover, develop and commercialize new ther-
apies: Chemistry; biotherapeutics; xRNA; radioligand
therapy; and gene and cell therapy.
• Priority geographies which, taken together, account
for the majority of the forecast growth in global health-
care spending: US, China, Germany and Japan. While
these are our priority countries, we will continue to
invest in other markets worldwide.
Our focus areas are supported by three strategic prior-
ities, which determine how we implement our strategy.
These three strategic priorities are:
• Deliver high-value medicines to accelerate growth.
Delivering new medicines for major diseases is at the
core of our purpose and value creation as a company.
We focus on high-value innovative medicines with the
potential to transform the treatment of diseases across
our five core therapeutic areas. To do this, we seek to
maximize the potential of our key in-market and launch
medicines, while finding new ways to deliver them to
as many people as possible and investing in R&D to
deliver the next generation of high-value therapies for
patients over the longer term. As part of our eorts, we
continue our longstanding commitment to reduce the
burden of infectious and tropical diseases that pre-
dominantly aect underserved populations in low- and
middle-income countries.

Item 5. Operating and Financial Review andProspects
51
• Embed operational excellence to deliver returns. We
aim to drive eciency and free up resources to invest
in innovation for patients. This also underpins our finan-
cial performance and makes us more agile; better able
to take quick decisions and scale the use of new tech-
nologies, with eective cooperation across our busi-
ness. In everything we do, we maintain high standards
of product quality and patient safety, while also work-
ing to reduce our environmental footprint.
• Strengthen our foundations by:
Unleashing the power of our people. We continue to
focus on culture as a key enabler of our strategy to
drive innovation and long-term performance. For us,
this is about building an agile, diverse workforce and
making sure we attract and retain the right talent for
the future.
Scaling data science and technology. We are investing
in data science and technology to increase eciency,
support innovation, better respond to the needs of
patients and healthcare professionals, and ultimately
improve the way we develop and deliver our medicines.
Building trust with society. We aim to increase access
to our medicines for underserved populations around
the world and follow high standards of ethical behav-
ior wherever we operate.

Item 5. Operating and Financial Review andProspects
52
Results of operations
Financial year 2022 compared with 2021
Key figures
1
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions unless indicated otherwise) Dec ,
Dec ,
Net sales to third parties
–
Other revenues
Cost of goods sold –
–
–
Gross profit
–
Selling, general and administration –
–
–
Research and development –
–
–
–
Other income
–
–
Other expense –
–
–
–
Operating income
–
–
of net sales to third parties
(Loss)/income from associated companies –
nm
nm
Interest expense –
–
–
–
Other financial income and expense
–
nm
nm
Income before taxes
–
–
Income taxes –
–
Net income
–
–
Attributable to:
Shareholders of Novartis AG
–
–
Non-controlling interests
–
nm
nm
Basic earnings per share (USD)
–
–
Net cash flows from operating activities
–
Free cash flow
–
1
For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS measures as defined by Novartis.”
nm = not meaningful

Item 5. Operating and Financial Review andProspects
53
Group overview
Net sales to third parties for Novartis were USD 50.5 bil-
lion, down 2% in USD reported terms and up 4% mea-
sured in constant currencies (cc) to remove the impact
of exchange rate movements. Sales growth was driven
by volume growth of 11 percentage points, mainly driven
by continued strong growth from Entresto, Kesimpta,
Kisqali, Pluvicto and Cosentyx. Generic competition had
a negative impact of 3 percentage points, mainly due to
Gilenya, Afinitor/Votubia, and Gleevec/Glivec. Pricing had
a negative impact of 4 percentage points. Sales in the
US were USD 17.7 billion (+5%) and in the rest of the world
USD 32.8 billion (–6%, +4% cc).
By division, Innovative Medicines delivered net sales
of USD41.3 billion (–2%, +4% cc) and Sandoz net sales
were USD9.2 billion (–4%, +4% cc).
In Emerging Growth Markets, which comprise all mar-
kets excluding the US, Canada, Western Europe, Japan,
Australia and New Zealand, sales to third parties were
USD13.5 billion (+2%, +9% cc) driven by China (USD3.1
billion) growing 2% (+6% cc).
Operating income was USD 9.2 billion (–21%, –13%
cc), mainly due to higher restructuring costs (USD 1.2 bil-
lion) primarily related to the implementation of the pre-
viously announced streamlined organizational model,
higher impairments (USD 1.0 billion), and lower divest-
ment gains (USD 0.6 billion). Operating income margin
was 18.2% of net sales, decreasing by 4.4 percentage
points (-3.8 percentage points cc).
Net income was USD 7.0 billion compared with USD
24.0 billion in the prior year, impacted by Roche income
in the prior year. Excluding the impact of Roche income,
net income declined –9% (cc). Earnings per share were
USD 3.19 compared with USD 10.71 in the prior year.
Excluding the impact of Roche income, EPS declined
–7% (cc).
Net cash flows from operating activities amounted to
USD 14.2 billion, compared with USD 15.1 billion in 2021.
This decrease was mainly due to unfavorable changes
in working capital and lower dividends from associated
companies (2021 included the USD 0.5 billion dividends
received from our investment in Roche, which was
divested in the fourth quarter of 2021), partly oset by
lower income taxes paid and favorable hedging results.
Free cash flow amounted to USD 11.9 billion (–10%
USD), compared with USD 13.3 billion in 2021, mainly due
to a decrease in net cash flows from operating activities
and lower divestment proceeds, partly oset by lower
purchases of property, plant and equipment.
We also present our core results
1
, which exclude the
impact of amortization, impairments, disposals, acquisi-
tions, restructurings and other significant items, to help
investors understand our underlying performance.
Core operating income was USD 16.7 billion (0%, +8%
cc), benefiting from higher sales, partly oset by higher
research and development (R&D) investments. Core
operating income margin was 33.0% of net sales,
increasing by 0.9 percentage points (+1.3 percentage
points cc).
Core net income was USD 13.4 billion (–5%, +3% cc)
as growth in core operating income was partly oset by
the loss of Roche core income. Excluding the impact of
Roche core income, core net income grew +11% (cc).
1
For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS
measures as defined by Novartis.”
Item 5. Operating and Financial Review andProspects
54
Net sales to third parties by segment
The following table provides an overview of net sales to third parties by segment:
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions) Dec ,
Dec ,
Innovative Medicines
–
Sandoz
–
Net sales to third parties
–
Innovative Medicines
The Innovative Medicines Division delivered net sales of
USD 41.3 billion (–2%, +4% cc) with volume contributing
12 percentage points to growth. Generic competition had
a negative impact of 4 percentage points. Pricing had a
negative impact of 4 percentage points. Sales in the US
were USD 15.9 billion (+6%) and in the rest of the world
USD 25.4 billion (–6%, +3% cc).
Sales growth was mainly driven by continued strong
growth from Entresto (USD 4.6 billion, +31%, +37% cc),
Kesimpta (USD 1.1 billion, +194%, +200% cc), Kisqali (USD
1.2 billion, +31%, +38% cc), Pluvicto (USD 271 million) and
Cosentyx (USD 4.8 billion, +1%, +5% cc), partly oset by
generic competition mainly for Gilenya, Afinitor/Votubia
and Gleevec/Glivec.
In the US (USD 15.9 billion +6%), sales growth was
mainly driven by Entresto, Kesimpta and Pluvicto, partly
oset by the impact of generic competition on Afinitor/
Votubia and Gilenya. In Europe (USD 13.6 billion, –9%,
+1% cc) sales growth was driven by Entresto, Kisqali and
Kesimpta, partly oset by increased generic competition
for Gilenya. Emerging Growth Markets grew +2% (+9%
cc), with China sales USD 2.9 billion (+3%, +7% cc) driven
by Cosentyx.
The following table provides an overview of net sales
to third parties by core therapeutic area; other promoted
brands; and established brands in the Innovative
Medicines Division:
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions) Dec ,
Dec ,
Cardiovascular
Immunology
Neuroscience
Solid Tumors
Hematology
Other Promoted Brands
–
–
Total Promoted Brands
Established Brands
–
–
Total Innovative Medicines
–
1
Reclassified to reflect the new Innovative Medicines divisional structures announced on April 4, 2022
Item 5. Operating and Financial Review andProspects
55
The following table provides the top 20 Innovative Medicines Division product net sales to third parties in 2022 as
well as the change compared with 2021:
US Rest of world Tot al
Brand classification by
therapeutic area, other
promoted brands or
change
change
change
change
change
Brands established brands Key indications USD m
USD/cc
USD m
USD
cc
USD m
USD
cc
Cosentyx Immunology Psoriasis (PsO),
–
ankylosing spondylitis
(AS), psoriatic arthritis
(PsA), non-radiographic
axial spondyloarthritis
(nr-axSPA)
Entresto Cardiovascular Chronic heart failure,
hypertension
Promacta/Revolade Hematology Immune
–
thrombocytopenia (ITP),
severe aplastic anemia (SAA)
Gilenya Neuroscience Relapsing multiple sclerosis
–
–
–
–
–
(RMS)
Tasigna Hematology Chronic myeloid leukemia
–
–
–
–
–
(CML)
Lucentis Other Promoted Age-related
–
–
–
–
Brands macular degeneration (AMD),
diabetic macular edema (DME),
retinal vein occlusion (RVO)
Tafinlar + Mekinist Solid Tumors BRAF V+ metastatic
adjuvant melanoma,
advanced non-small cell
lung cancer (NSCLC),
tumor agnostic with
BRAF mutation indication
Jakavi Hematology Myelofibrosis (MF),
–
–
polycytomia vera (PV),
graft-versus-host disease
(GvHD)
Zolgensma Neuroscience Spinal muscular atrophy
–
(SMA)
Xolair
Immunology Severe allergic asthma (SAA),
–
–
chronic spontaneous urticaria
(CSU), nasal polyps
Sandostatin Established Brands Carcinoid tumors,
–
–
–
–
–
acromegaly
Kisqali Solid Tumors HR+/HER-
metastatic breast cancer
Ilaris Immunology Auto-inflammatory (CAPS,
TRAPS, HIDS/MKD, FMF,
SJIA, AOSD, gout)
Kesimpta Neuroscience Relapsing-remitting
nm
nm
multiple sclerosis (RRMS)
Galvus Group Established Brands Type diabetes
–
–
–
–
Gleevec/Glivec Established Brands Chronic myeloid
–
–
–
–
–
leukemia (CML),
gastrointestinal stromal
tumors (GIST)
Exforge Group Established Brands Hypertension
–
–
–
–
Diovan Group Established Brands Hypertension
–
–
–
–
Kymriah Hematology r/r pediatric and young
–
–
–
–
adults acute lymphoblastic
leukemia (ALL), diuse large
B-cell lymphoma (DLBCL),
follicular lymphoma (FL)
Afinitor/Votubia Established Brands Breast cancer/
–
–
–
–
–
tuberous sclerosis complex
(TSC)
Top brands total
–
–
Rest of portfolio
–
–
Total division net
sales to third parties
–
–
1
Net sales to third parties reflect Xolair sales for all indications.
2
For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS measures as defined by Novartis.”
nm = not meaningful

Item 5. Operating and Financial Review andProspects
56
For the table providing the top 20 Innovative Medicines Division product net sales to third parties in 2021, see “Item
18. Financial statements—Note 3. Segmentation of key figures 2022, 2021 and 2020.”
For information about the approved indications for certain products described, see “Item 4. Information on the
Company—Item 4.B Business overview—Innovative Medicines— Innovative Medicines Division products.”
CARDIOVASCULAR
Sales in the Cardiovascular therapeutic area were USD
4.8 billion (+34%, +40% cc), sales growth mainly driven
by Entresto.
Entresto (USD 4.6 billion, +31%, +37% cc) sustained
robust demand-led growth, with increased patient share
across all geographies. Guidelines position Entresto as
the first choice RASi versus ACEi/ARB in patients with
HFrEF. Entresto benefits from the adoption of guideline
directed medical therapy for these patients in all geog-
raphies. In the US, Entresto benefits from being added
to guidelines for patients with HFpEF (with LVEF below
normal). In China, Entresto has been listed in the National
Reimbursement Drug List (NRDL) for both HFrEF and
hypertension, eective January 2022. In China and
Japan, Entresto volume growth is fueled by increased
penetration in hypertension in addition to growth in heart
failure. It is estimated that around 10 million patients are
on treatment with Entresto.
Leqvio (USD 0.1 billion) launch in the US and other
markets is ongoing, with focus on patient on-boarding,
removing access hurdles and enhancing medical edu-
cation. Leqvio is the first and only small interfering RNA
(siRNA) therapy to lower low-density lipoprotein choles-
terol approved in the US and was launched in January
2022. In the US, Leqvio is covered at or near label for
76% of patients eleven months after launch. Leqvio in
the US has been assigned a unique Healthcare Common
Procedure Coding System code (J-code) and average
sales price. Leqvio is now approved in 70 countries.
Novartis obtained global rights to develop, manufacture
and commercialize Leqvio under a license and collabo-
ration agreement with Alnylam Pharmaceuticals.
IMMUNOLOGY
Sales in the Immunology therapeutic area reached USD
7.3 billion (+1%, +7% cc), sales growth was mainly driven
by Cosentyx and Ilaris.
Cosentyx (USD 4.8 billion, +1%, +5% cc) sales grew
in Emerging Growth Markets, Europe and Japan, partly
oset by decline in the US due to higher revenue deduc-
tions. In China, Cosentyx growth was fueled by increased
biologic uptake and inclusion in approximately 1,900 hos-
pital listings. Since initial approval in 2015, Cosentyx has
proven its sustained ecacy and consistent safety pro-
file across five systemic inflammatory conditions and has
treated more than 960,000 patients worldwide.
Xolair (USD 1.4 billion, –4%, +6% cc) sales grew (cc)
in Emerging Growth Markets, Europe and Japan. Novartis
co-promotes Xolair with Genentech in the US and shares
a portion of revenue as operating income but does not
record any US sales.
Ilaris (USD 1.1 billion, +7%, +15% cc) showed contin-
ued growth across all geographies. Contributors to
growth include the adult-onset Still’s disease indication,
together with the other adult rheumatology indications
in the US and Europe, as well as strong performance for
the Periodic Fevers Syndrome indications in Japan.
NEUROSCIENCE
Sales in the Neuroscience therapeutic area were USD
5.1 billion (+1%, +5% cc), sales growth (cc) mainly driven
by Kesimpta, which was partly oset by sales decline of
Gilenya.
Gilenya (USD 2.0 billion, –28%, –24% cc) sales
declined mainly in Europe and in the US due to generic
pressure.
Zolgensma (USD 1.4 billion, +1%, +5% cc) has been
approved in 47 countries to date. As this represents most
major markets, sales growth is now mainly driven by the
Incident patient population where we’ve seen double
digit growth in 2022. Access pathways are now in place
in 35 countries with negotiations ongoing in additional
markets.
Kesimpta (USD 1.1 billion, +194%, +200% cc) showed
strong sales growth driven by launch momentum across
all geographies. Kesimpta is a targeted B-cell therapy
that can deliver powerful and sustained high ecacy,
with a favorable safety and tolerability profile and the
flexibility of an at home self-administration for a broad
population of RMS patients. Kesimpta is now approved
in 80 countries with more than 36,000 patients treated.
Mayzent (USD 0.4 billion, +27%, +32% cc) sales grew
across all geographies in MS patients showing signs of
progression despite being on other treatments. Mayzent
is the first and only oral disease-modifying therapy stud-
ied and proven to delay disease progression in a broad
SPMS patient population.
Aimovig (USD 0.2 billion, +1%, +11% cc) sales grew in
Europe and Emerging Growth Markets. Aimovig is reim-
bursed in 32 markets and has been prescribed to over
759,000 patients worldwide. Earlier this year, Aimovig
was submitted for approval in China. In October 2022,
Novartis reached an agreement in Germany by which
Aimovig is reimbursed as a 1
st
line prophylactic migraine
treatment based on the HERMES trial.
SOLID TUMORS
Sales in the Solid Tumors therapeutic area were USD 4.7
billion (+15%, +21% cc), sales growth mainly driven by
Kisqali, Pluvicto and Tafinlar + Mekinist.
Tafinlar + Mekinist (USD 1.8 billion, +5%, +11% cc)
sales grew across all geographies, driven by demand in
BRAF+ adjuvant melanoma and NSCLC indications,
while maintaining demand in the highly competitive
BRAF+ metastatic melanoma market. Tafinlar + Mekinist
remains the worldwide targeted therapy leader in BRAF+
melanoma. Following FDA approval in late June, Tafinlar
+ Mekinist is the first and only therapy with a tumor-ag-
nostic indication for adult and pediatric patients with
solid tumors that have a BRAF V600E mutation, which
drives tumor growth in more than 20 dierent tumor
types.
Kisqali (USD 1.2 billion, +31%, +38% cc) sales grew
strongly across all geographies, based on increasing rec-
ognition of its overall survival and quality of life benefits
in HR+/HER2- advanced breast cancer. It is a CDK4/6

Item 5. Operating and Financial Review andProspects
57
inhibitor with proven overall survival benefit across all
three Phase III trials of the MONALEESA program
regardless of menopausal status, line of therapy, site and
number of metastases, endocrine resistance, or endo-
crine partner.
Votrient (USD 0.5 billion, –18%, –13% cc) declined due
to increased competition, especially from immuno-on-
cology agents in metastatic renal cell carcinoma.
Lutathera (USD 0.5 billion, –1%, +3% cc) sales grew
(cc) in Europe and Japan, partly oset by decline in the
US. There are approximately 500 centers actively treat-
ing patients globally. In the second quarter of 2022, there
was a temporary suspension in manufacturing during the
quarter; production and deliveries of patient doses
resumed in early June.
Piqray (USD 0.4 billion, +13%, +14% cc) sales grew
mainly in the US, benefiting from indication expansion
into PIK3CA-related overgrowth spectrum (PROS).
Piqray is the first and only therapy specifically developed
for the approximately 40% of HR+/HER2- advanced
breast cancer patients who have a PIK3CA mutation,
which is associated with a worse prognosis.
Pluvicto (USD 0.3 billion) launch is progressing well,
with more than 160 active centers ordering. Pluvicto is
the first and only radioligand therapy approved by the
FDA for the treatment of progressive, PSMA-positive
metastatic castration-resistant prostate cancer, who
have already been treated with other anticancer treat-
ments (ARPI and taxane-based chemotherapy).
Tabrecta (USD 0.1 billion, +48%, +48% cc) sales grew
across all geographies, as the first therapy approved by
the FDA to specifically target metastatic NSCLC with a
mutation that leads to MET exon 14 skipping (METex14).
HEMATOLOGY
Sales in the Hematology therapeutic area were USD 6.5
billion (0%, +7% cc), sales growth (cc) mainly driven by
Promacta/Revolade, Jakavi and Scemblix.
Promacta/Revolade (USD 2.1 billion, +4%, +9% cc)
growth was driven by the US, Europe and Emerging
Growth Markets, partly oset by decline in Japan. Sales
growth was driven by increased use in second-line per-
sistent and chronic immune thrombocytopenia and as
first-line and/or second-line treatment for severe aplas-
tic anemia.
Tasigna (USD 1.9 billion, –7%, –1% cc) sales declined
in Europe, Japan and the US, partly oset by growth in
Emerging Growth Markets.
Jakavi (USD 1.6 billion, –2%, +9% cc) sales grew (cc)
in Europe, Emerging Growth Markets, Japan, driven by
strong demand in both the myelofibrosis and polycythe-
mia vera indications. In May, EC approved Jakavi for the
treatment of patients aged 12 years and older with acute
or chronic GvHD who have inadequate response to cor-
ticosteroids or other systemic therapies.
Kymriah (USD 0.5 billion, –9%, –2% cc) sales declined
in the US and Europe due to lower DLBCL demand in
both geographies and was partly oset by growth in
Emerging Growth Markets and Japan. In May, EC and
FDA approved Kymriah for the treatment of adult patients
with relapsed or refractory (r/r) follicular lymphoma (FL)
after two or more lines of systemic therapy.
Adakveo (USD 0.2 billion, +18%, +19% cc) continued
to grow worldwide, reaching more than 11,800 patients
with vaso-occlusive crises caused by sickle cell disease
to date.
Scemblix (USD 0.1 billion) continued its strong launch
uptake in the US, with launches underway in EU and
Japan, demonstrating the high unmet need in CML, par-
ticularly patients previously treated with 2 or more tyro-
sine kinase inhibitors, or with the T315I mutation. In Octo-
ber 2022, US FDA converted the accelerated approval
of Scemblix to a full approval, confirming the clinical ben-
efit after longer exposure.
OTHER PROMOTED BRANDS
Sales for Other Promoted Brands were USD 3.1 billion
(–9%, –1% cc).
Lucentis (USD 1.9 billion, –13%, –4% cc) sales declined
in Japan and Europe mainly due to competition, which
was partly oset by growth in Emerging Growth Markets.
Xiidra (USD 0.5 billion, +4%, +4% cc) sales grew
mainly in the US.
Ultibro Group (USD 0.5 billion, –18%, –9% cc) sales
declined in Europe and Emerging Growth Markets due
to competition and was partly oset by growth in Japan.
Ultibro Group consists of Ultibro Breezhaler, Seebri Bree-
zhaler and Onbrez Breezhaler.
Beovu (USD 0.2 billion, +9%, +18% cc) sales grew in
Europe, Emerging Growth Markets and Japan, partly o-
set by decline in the US. Beovu received approval for dia-
betic macular edema (DME) in the EU in the first quarter
of 2022, and in the US in the second quarter of 2022.
ESTABLISHED BRANDS
The Established Brands had sales of USD 9.9 billion
(–19%, –13% cc).
Sandostatin (USD 1.2 billion, –12%, –10% cc) declined
across all geographies due to ongoing competitive pres-
sure, including generic competition ex-US.
Galvus Group (USD 0.9 billion, –21%, –12% cc)
declined in Japan, Europe and Emerging Growth Mar-
kets.
Gleevec/Glivec (USD 0.7 billion, –27%, –22% cc)
declined due to increased generic competition.
Exforge Group (USD 0.7 billion, –18%, –12% cc)
declined across all geographies.
Diovan Group (USD 0.7 billion, –16%, –9% cc) declined
in Emerging Growth Markets, Japan and Europe.
Afinitor/Votubia (USD 0.5 billion, –45%, –41% cc)
declined in the US and Europe driven by generic com-
petition.
Voltaren/Cataflam (USD 0.3 billion, –10%, 0% cc)
sales were stable (cc).
Zortress/Certican (USD 0.3 billion, –24%, –14% cc)
declined in the US and Japan.
Exjade/Jadenu (USD 0.3 billion, –43%, –38% cc)
declined due to pressure from generic competition.
Neoral/Sandimmun(e) (USD 0.3 billion, –16%, –8% cc)
declined across all geographies.

Item 5. Operating and Financial Review andProspects
58
Sandoz
Sandoz net sales were USD 9.2 billion (–4%, +4% cc)
with volume contributing 10 percentage points to growth.
Pricing had a negative impact of 6 percentage points.
Sales in Europe were USD 4.9 billion (–7%, +4% cc),
in the US USD 1.8 billion (–4%) in Asia/Africa/Australasia
USD 1.6 billion (–3%, +6% cc) and in Canada and Latin
America USD 969 million (+11%, +15% cc) driven by vol-
ume increases and tender wins.
The following table provides an overview of net sales
to third parties by business franchise in the Sandoz Divi-
sion:
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions) Dec ,
Dec ,
Retail Generics
–
Biopharmaceuticals
–
Anti-Infectives
(partner label/API)
–
–
Total Sandoz
–
1
Sandoz total anti-infectives net sales to third parties amounted to USD 1.2 billion
(2021: USD 1.1 billion; 2020: USD 1.2 billion), of which USD 777 million (2021: USD 707
million; 2020: USD 694 million) is sold through the Retail Generics business franchise
and USD 380 million (2021: USD 423 million; 2020: USD 474 million) is sold to other
third-party companies through the Anti-Infectives business franchise.
Retail Generics
In Retail Generics, Sandoz develops, manufactures and
markets finished dosage forms of small molecule
pharmaceuticals for sale to third parties across a broad
range of therapeutic areas, including finished dosage
form of anti-infectives sold to third parties.
Retail sales were USD 6.8 billion (–4%, +4% cc), grow-
ing across all regions ex-US.
Biopharmaceuticals
In Biopharmaceuticals, Sandoz develops, manufactures
and markets protein- and other biotechnology-based
products, including biosimilars, and provides biotechnol-
ogy manufacturing services to other companies. The
Biopharmaceuticals business also includes Glatopa, a
generic version of Copaxone
®
, which treats relapsing
forms of multiple sclerosis and is marketed in the US.
Global sales of Biopharmaceuticals (biosimilars, bio-
pharmaceutical contract manufacturing and Glatopa)
grew to USD 2.1 billion (–1%, +9% cc), growing across all
regions.
Anti-Infectives
In Anti-Infectives, Sandoz manufactures and supplies
active pharmaceutical ingredients and intermediates,
mainly antibiotics, for internal use by Retail Generics and
for sale to third-party customers.
Total Anti-Infectives sales were USD 1.2 billion (+2%,
+10% cc) of which USD 777 million were sold through the
Retail Generics business franchise and USD 380 million
were sold to other third-party companies through the
Anti-Infectives business franchise. The sales of the
Anti-Infectives business franchise declined mainly due
to product discontinuations and supply challenges.
Item 5. Operating and Financial Review andProspects
59
Operating income
The following table provides an overview of operating income by segment:
of
of
Change in
net sales
net sales
Change
constant
Year ended
to third
Year ended
to third
in USD
currencies
(USD millions) Dec ,
parties
Dec ,
parties
Innovative Medicines
–
–
Sandoz
–
–
Corporate –
–
–
–
Operating income
–
–
Operating income was USD 9.2 billion (–21%, –13% cc), mainly due to higher restructuring (USD 1.2 billion) primar-
ily related to the implementation of the previously announced streamlined organizational model, higher impairments
(USD 1.0 billion) and lower divestment gains (USD 0.6 billion). Operating income margin was 18.2% of net sales,
decreasing by 4.4 percentage points (-3.8 percentage points cc).
Core operating income key figures
1
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions unless indicated otherwise) Dec ,
Dec ,
Core gross profit
–
Selling, general and administration –
–
–
Research and development –
–
–
–
Other income
–
–
Other expense –
–
Core operating income
As of net sales to third parties
1
For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS measures as defined by Novartis.”
The adjustments made to operating income to arrive at
core operating income amounted to USD7.5 billion (com-
pared with USD4.9 billion in the prior year). For details,
please see “—Non-IFRS measures as defined by
Novartis—2022 and 2021 reconciliation from IFRS
results to core results.”
Core operating income was USD 16.7 billion (0%, +8%
cc) benefiting from higher sales, partly oset by higher
R&D investments. Core operating income margin was
33.0% of net sales, increasing by 0.9 percentage points
(+1.3 percentage points cc).
The following table provides an overview of core operating income by segment:
of
of
Change in
net sales
net sales
Change
constant
Year ended
to third
Year ended
to third
in USD
currencies
(USD millions) Dec ,
parties
Dec ,
parties
Innovative Medicines
Sandoz
–
–
Corporate –
–
Core operating income
Innovative Medicines
Operating income was USD 8.8 billion (–18%, –9% cc),
driven by higher impairments, restructuring, lower divest-
ment gains and higher R&D expenses, partly oset by
higher gross margin. Operating income margin was
21.3% of net sales, decreasing 4.2 percentage points
(-3.4 percentage points in cc).
Core adjustments were USD 6.5 billion, mainly due
to amortization, impairments and restructuring, com-
pared to USD 4.5 billion in prior year. Core adjustments
increased compared to prior year, mainly due to higher
impairments and restructuring.
Core operating income was USD 15.2 billion (0%, +8%
cc), mainly driven by higher gross margin, partly oset

Item 5. Operating and Financial Review andProspects
60
by higher R&D investments. Core operating income mar-
gin was 36.9% of net sales, increasing 0.7 percentage
points (+1.3 percentage points cc). Revenues as a per-
centage of sales increased by 0.1 percentage points (cc).
Core cost of goods sold as a percentage of sales was
in line with the prior year. Core R&D expenses as a per-
centage of net sales increased by 0.2 percentage points
(cc). Core selling, general and administration (SG&A)
expenses as a percentage of net sales decreased by 1.4
percentage points (cc). Core other income and expense
as a percentage of net sales was in line with the prior
year.
Sandoz
Operating income was USD 1.4 billion (–10%, –2% cc),
with the decline mainly due to higher SG&A investments
to drive higher sales and inflationary pressures on input
costs, which were partly oset by higher sales. Operat-
ing income margin was 15.7% of net sales, decreasing
by 0.9 percentage points (-1.0 percentage points in cc).
Core adjustments were USD 455 million, including
USD 221 million of amortization. Prior year core adjust-
ments were USD 464 million, including USD 236 million
of amortization.
Core operating income was USD 1.9 billion (–8%, –1%
cc), with the decline mainly due to higher SG&A, partly
oset by higher sales. Core operating margin was 20.6%
of net sales, decreasing by 0.8 percentage points (-1.1
percentage points cc). Core gross margin as a percent-
age of sales decreased by 0.3 percentage points (cc),
due to higher inflation and input costs. Core R&D
expenses as a percentage of net sales decreased by 0.5
percentage points (cc). Core SG&A expenses increased
by 0.9 percentage points (cc). Core other income and
expense decreased the margin by 0.4 percentage points
(cc).
Corporate income and expense, net
Corporate income and expense, which includes the cost
of Group headquarter and coordination functions,
amounted to an expense of USD 1.0 billion, compared to
an expense of USD 599 million in 2021, mainly driven by
higher restructuring costs, lower contributions from the
Novartis Venture Fund and prior year income from a fair
value adjustment on contingent receivables related to
intellectual property rights, partly oset by prior year
adjustments to provisions on M&A transactions.
Innovative Medicines Division research and development
The following table provides an overview of the reported and core research and development expense of the
Innovative Medicines Division:
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions unless indicated otherwise) Dec ,
Dec ,
Research and exploratory development –
–
Confirmatory development –
–
–
–
Total Innovative Medicines Division research and development expense –
–
–
–
As of Innovative Medicines net sales to third parties
Core research and exploratory development
–
–
–
Core confirmatory development
–
–
–
–
Total core Innovative Medicines Division research and development expense –
–
–
–
As of Innovative Medicines net sales to third parties
1
Core results exclude impairments, amortization and certain other items. For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS measures as defined
by Novartis.”
Innovative Medicine Division research and exploratory
development expense decreased by 8% (+6% cc) to USD
2.9 billion. Confirmatory development expense amounted
to USD 6.2 billion, increasing by 15% (–20% cc) versus
prior year mainly due to higher impairment charges and
higher investments in development to support recently
acquired assets.
Total core research and development expense in the
Innovative Medicine Division as a percentage of sales
increased by 0.6 percentage points (+0.2 percentage
points cc) to 20.0% of net sales, mainly driven by higher
investments in recently acquired assets.

Item 5. Operating and Financial Review andProspects
61
Non-operating income and expense
The term “non-operating income and expense” includes all income and expense items outside operating income.
The following table provides an overview of non-operating income and expense:
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions unless indicated otherwise) Dec ,
Dec ,
Operating income
–
–
(Loss)/income from associated companies –
nm
nm
Interest expense –
–
–
–
Other financial income and expense
–
nm
nm
Income before taxes
–
–
Income taxes –
–
Net income
–
–
Attributable to:
Shareholders of Novartis AG
–
–
Non-controlling interests
–
nm
nm
Basic earnings per share (USD)
–
–
nm = not meaningful
Income from associated companies
Income from associated companies was a loss of USD
9 million compared to an income of USD 15.3 billion in
prior year. This decrease was due to the divestment of
our investment in Roche that closed in the fourth quar-
ter of 2021 where a gain of USD 14.6 billion was recog-
nized.
Interest expense and other financial income and
expense
Interest expense amounted to USD 837 million, broadly
in line with prior year.
Other financial income and expense amounted to an
income of USD 20 million compared to an expense of
USD 80 million in the prior year, as higher interest income
was partly oset by financial expenses and currency
losses.
Income taxes
The tax rate was 16.9% compared to 8.1% in the prior
year period. In the prior year, the tax rate was impacted
by the Roche income from associated companies (includ-
ing the divestment gain recognized on the sale of our
investment in Roche in December 2021), the impact of
increases in uncertain tax positions and prior-year items.
For comparability, excluding these impacts, the prior year
tax rate would have been 16.8%, broadly in line with 16.9%
in the current year.
Net income
Net income was USD 7.0 billion (–71%, –67% cc), impacted
by Roche income in the prior year. Excluding the impact
of Roche income, net income declined –9% (cc).
Earnings per share
Basic earnings per share were USD 3.19 compared with
USD 10.71 in the prior year, mainly due to prior year Roche
income. Excluding the impact of Roche income, EPS
declined –7% (cc).

Item 5. Operating and Financial Review andProspects
62
Core non-operating income and expense
1
The following table provides an overview of core non-operating income and expense:
Change in
Change
constant
Year ended
Year ended
in USD
currencies
(USD millions unless indicated otherwise) Dec ,
Dec ,
Core operating income
Core (loss)/income from associated companies –
nm
nm
Core interest expense –
–
–
–
Core other financial income and expense
–
nm
nm
Core income before taxes
–
Core income taxes –
–
–
Core net income
–
Core basic earnings per share (USD)
–
nm = not meaningful
Core income from associated companies
Core income from associated companies was a loss of
USD 9 million compared with an income of USD 993 mil-
lion in prior year. This decrease was due to the divest-
ment of our investment in Roche that closed in the fourth
quarter of 2021.
Core interest expense and other financial income
and expense
Core interest expense amounted to USD 837 million,
broadly in line with prior year.
Core other financial income and expense amounted
to an income of USD 141 million compared to an expense
of USD 41 million in the prior year as higher interest
income was only partly oset by currency losses.
Core income taxes
The core tax rate (core taxes as a percentage of core
income before tax) was 16.3% compared to 15.8% in the
prior year. For comparability, excluding Roche Income
from associated companies (divested in December
2021), the prior year core tax rate would have been 16.7%
compared to 16.3% in the current year, decreasing mainly
as a result of a change in core profit mix.
Core net income
Core net income was USD 13.4 billion (–5%, +3% cc) as
growth in core operating income was partly oset by the
loss of Roche core income. Excluding the impact of
Roche core income, core net income grew +11% (cc).
Core earnings per share
Core EPS was USD 6.12 (–3%, +6% cc), benefiting from
lower weighted average number of shares outstanding.
Excluding the impact of Roche core income, core EPS
grew +14% (cc).
1
For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS
measures as defined by Novartis.”

Item 5. Operating and Financial Review andProspects
63
Results of operations excluding Roche investment impacts
To enhance investors’ understanding of the Group’s performance in comparison with the prior year, the following
table provides a comparison of our 2022 published IFRS results and non-IFRS measures core results and free cash
flow with the 2021 results, excluding the impacts related to our Roche investment, due to its divestment.
Excluding Roche investment impacts
2
Year ended
Year ended
change
change
Dec ,
Dec ,
USD
cc
Operating income
–
–
Loss from associated companies –
–
nm
nm
Interest expense –
–
–
–
Other financial income and expense
–
nm
nm
Income taxes –
–
Net income
–
–
Basic earnings per share (USD)
–
–
Net cash flows from operating activities
–
Free cash flow
–
Core
Core operating income
Core net income
Core basic earnings per share (USD)
1
For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS measures as defined by Novartis.”
2
For a reconciliation of 2021 IFRS results and non-IFRS measures core results and free cash flow to exclude the impacts of the 2021 divestment of our Roche investment, see “—
Non-IFRS measures as defined by Novartis.”
nm = not meaningful

Item 5. Operating and Financial Review andProspects
64
Factors aecting comparability of year-on-year results
of operations
Significant transactions
in 2022 and 2021
The comparability of the year-on-year results of our
operations for the total Group can be significantly
aected by acquisitions and divestments. As part of our
long-term strategy to focus Novartis as a leading
medicines company, we announced and/or completed
several acquisitions and divestments during 2022 and
2021.
A detailed description of significant transactions in
2022 and 2021, can be found in “Item 18. Financial State-
ments—Note 2. Significant transactions.”
Internal control over financial reporting
The Group’s management has assessed the eective-
ness of internal control over financial reporting. The
Group’s independent statutory auditor also issued an
opinion on the eectiveness of internal control over
financial reporting. Both the Group’s management and
its external auditors concluded that the Group main-
tained, in all material respects, eective internal control
over financial reporting as of December 31, 2022. For
more details, see “Item 15. Controls and Procedures.”
Approach to risk management
See “Item 6. Directors, Senior Management and Employ-
ees—Item 6.C Board practices—Corporate gover-
nance—Information and control systems—Risk
management” and “Item 18. Financial Statements—Note
29. Financial instruments – additional disclosures.”
Non-IFRS measures as defined by Novartis
Novartis uses certain non-IFRS metrics when measur-
ing performance, especially when measuring cur-
rent-year results against prior periods, including core
results, constant currencies and free cash flow.
Despite the use of these measures by management
in setting goals and measuring the Group’s performance,
these are non-IFRS measures that have no standardized
meaning prescribed by IFRS. As a result, such measures
have limits in their usefulness to investors.
Because of their non-standardized definitions, the
non-IFRS measures (unlike IFRS measures) may not be
comparable to the calculation of similar measures of
other companies. These non-IFRS measures are pre-
sented solely to permit investors to more fully understand
how the Group’s management assesses underlying per-
formance. These non-IFRS measures are not, and should
not be viewed as, a substitute for IFRS measures, and
should be viewed in conjunction with IFRS financials.
As an internal measure of Group performance, these
non-IFRS measures have limitations, and the Group’s
performance management process is not solely
restricted to these metrics.

Item 5. Operating and Financial Review andProspects
65
Core results
The Group’s core results – including core operating
income, core net income and core earnings per share –
exclude fully the amortization and impairment charges
of intangible assets, excluding software, net gains and
losses on fund investments and equity securities valued
at fair value through profit and loss, and certain acquisi-
tion- and divestment-related items. The following items
that exceed a threshold of USD 25 million are also
excluded: integration- and divestment-related income
and expenses; divestment gains and losses; restructur-
ing charges/releases and related items; legal-related
items; impairments of property, plant and equipment,
software, and financial assets, and income and expense
items that management deems exceptional and that are
or are expected to accumulate within the year to be over
a USD25 million threshold.
Novartis believes that investor understanding of the
Group’s performance is enhanced by disclosing core
measures of performance, since core measures exclude
items that can vary significantly from year to year, they
enable better comparison of business performance
across years. For this same reason, Novartis uses these
core measures in addition to IFRS and other measures
as important factors in assessing the Group’s perfor-
mance.
The following are examples of how these core measures
are utilized:
• In addition to monthly reports containing financial infor-
mation prepared under International Financial Report-
ing Standards (IFRS), senior management receives a
monthly analysis incorporating these core measures.
• Annual budgets are prepared for both IFRS and core
measures.
As an internal measure of Group performance, the core
results measures have limitations, and the Group’s per-
formance management process is not solely restricted
to these metrics. A limitation of the core results mea-
sures is that they provide a view of the Group’s opera-
tions without including all events during a period, such
as the eects of an acquisition, divestment, or amortiza-
tion/impairments of purchased intangible assets, impair-
ments to property, plant and equipment and restructur-
ings and related items.
Constant currencies
Changes in the relative values of non-US currencies to
the US dollar can aect the Group’s financial results and
financial position. To provide additional information that
may be useful to investors, including changes in sales
volume, we present information about our net sales and
various values relating to operating and net income that
are adjusted for such foreign currency eects.
Constant currency calculations have the goal of elim-
inating two exchange rate eects so that an estimate
can be made of underlying changes in the consolidated
income statement excluding the impact of fluctuations
in exchanges rates:
• The impact of translating the income statements of
consolidated entities from their non-USD functional
currencies to USD
• The impact of exchange rate movements on the major
transactions of consolidated entities performed in cur-
rencies other than their functional currency.
We calculate constant currency measures by translating
the current year’s foreign currency values for sales and
other income statement items into USD (excluding the
IAS 29 “Financial Reporting in Hyperinflationary Econo-
mies” adjustments to the local currency income state-
ments of subsidiaries operating in hyperinflationary
economies), using the average exchange rates from the
prior year and comparing them to the prior year values
in USD.
We use these constant currency measures in evalu-
ating the Group’s performance, since they may assist us
in evaluating our ongoing performance from year to year.
However, in performing our evaluation, we also consider
equivalent measures of performance that are not aected
by changes in the relative value of currencies.
Growth rate calculation
For ease of understanding, Novartis uses a sign conven-
tion for its growth rates such that a reduction in operat-
ing expenses or losses compared with the prior year is
shown as a positive growth.
Free cash flow
Novartis defines free cash flow as net cash flows from
operating activities and cash flows from investing activ-
ities associated with purchases and sales of property,
plant and equipment, of intangible assets, of financial
assets and of other non-current assets. Excluded from
free cash flow are cash flows from investing activities
associated with acquisitions and divestments of busi-
nesses and of interests in associated companies, pur-
chases and sales of marketable securities, commodities,
time deposits and net cash flows from financing activi-
ties.
Free cash flow is a non-IFRS measure and is not
intended to be a substitute measure for net cash flows
from operating activities as determined under IFRS. Free
cash flow is presented as additional information because
management believes it is a useful supplemental indica-
tor of the Group’s ability to operate without reliance on
additional borrowing or use of existing cash. Free cash
flow is a measure of the net cash generated that is avail-
able for investment in strategic opportunities, returning
to shareholders and for debt repayment. Free cash flow
is a non-IFRS measure, which means it should not be
interpreted as a measure determined under IFRS.

Item 5. Operating and Financial Review andProspects
66
Additional information
NET DEBT
Novartis calculates net debt as current financial debts
and derivative financial instruments plus non-current
financial debt less cash and cash equivalents and mar-
ketable securities, commodities, time deposits and deriv-
ative financial instruments.
Net debt is presented as additional information
because it sets forth how management monitors net debt
or liquidity and management believes it is a useful sup-
plemental indicator of the Group’s ability to pay divi-
dends, to meet financial commitments, and to invest in
new strategic opportunities, including strengthening its
balance sheet.
For the table that shows the Group’s net debt, see
“— Item 5.B Liquidity and capital resources — Group
liquidity, financial debts and net debt.”
EBITDA
Novartis defines earnings before interest, tax, depreci-
ation and amortization (EBITDA) as operating income,
excluding depreciation of property, plant and equipment,
depreciation of right-of-use assets, amortization of intan-
gible assets, and impairments of property, plant and
equipment, right-of-use assets and of intangible assets.
(USD millions)
Operating income
Depreciation of property,
plant and equipment
Depreciation of
right-of-use assets
Amortization of intangible
assets
Impairments of property,
plant and equipment, right-of-use assets and
intangible assets
EBITDA
1
There were no impairments of right-of-use assets in 2021.
ENTERPRISE VALUE
Enterprise value represents the total amount that share-
holders and debt holders have invested in Novartis, less
the Group’s liquidity.
(USD millions) Dec ,
Dec ,
Market capitalization
Non-controlling interests
Non-current financial debts
Current financial debts and
derivative financial instruments
Marketable securities,
commodities, time deposits
and derivative financial
instruments –
–
Cash and cash equivalents –
–
Enterprise value

Item 5. Operating and Financial Review andProspects
67
Reconciliation from IFRS results to core results
The following tables provide an overview of the reconciliation from IFRS results to core results:
2022 and 2021 reconciliation from IFRS results to core results
Innovative Medicines Sandoz Corporate Group
(USD millions unless indicated otherwise)
IFRS operating income
–
–
Amortization of intangible assets
Impairments
Intangible assets
Property, plant and equipment related to the Group-wide
rationalization of manufacturing sites
–
Other property, plant and equipment
Total impairment charges
Acquisition or divestment of businesses and related items
- Income
–
–
–
–
–
- Expense
Total acquisition or divestment of
businesses and related items, net
–
–
Other items
Divestment gains –
–
–
–
–
–
–
Financial assets – fair value adjustments
–
–
Restructuring and related items
- Income –
–
–
–
–
–
–
–
- Expense
Legal-related items
- Income –
–
–
–
- Expense
Additional income –
–
–
–
–
–
–
–
Additional expense
Total other items
–
Total adjustments
–
Core operating income
–
–
as of net sales
(Loss)/income from associated companies –
–
–
Core adjustments to income from associated companies, net of tax
–
–
Interest expense
–
–
Other financial income and expense
–
Core adjustments to other financial income and expense
Income taxes, adjusted for above items (core income taxes)
–
–
Core net income
Core net income attributable to shareholders of Novartis AG
Core basic EPS (USD)
1
Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.

Item 5. Operating and Financial Review andProspects
68
2022 and 2021 reconciliation from IFRS results to core results – Group
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions unless indicated otherwise) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
Operating income
Income before taxes
Income taxes
–
–
Net income
Basic EPS (USD)
The following are adjustments to arrive at core gross profit
Other revenues
–
Cost of goods sold –
–
The following are adjustments to arrive at core operating income
Selling, general and administration –
–
Research and development –
–
–
Other income
–
–
–
Other expense –
–
The following are adjustments to arrive at core income before taxes
Other financial income and expense
1
Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets;
research and development includes the amortization of acquired rights for technologies
2
Impairments: cost of goods sold, research and development and other expense include impairment charges related to intangible assets; other income and other expense include
net impairment charges related to property, plant and equipment
3
Acquisition or divestment of businesses and related items, including restructuring and integration charges: other income and other expense include transitional service fee income
and charges related to divestments; other income also includes adjustments to provisions; other expense includes stamp duties related to an acquisition
4
Other items: other revenues includes a net income from an outlicensing agreement; cost of goods sold, selling, general and administration, research and development, other
income and other expense include restructuring income and charges related to the restructuring initiative to implement a new streamlined organizational model, the Sandoz
strategic review, the Group-wide rationalization of manufacturing sites and other net restructuring charges and related items; cost of goods sold, selling, general and
administration, research and development and other expense include adjustments to provisions and related items; cost of goods sold and research and development also include
contingent consideration adjustments; other income and other expense include fair value adjustments and divestment gains and losses on financial assets and legal-related items;
other income also includes gains from the divestment of products and property, curtailment gains and an adjustment to an environmental provision; other expense includes a
reversal of an accrual and other costs and items; other financial income and expense includes the monetary loss on the restatement of non-monetary items for subsidiaries in
hyperinflationary economies and a revaluation impact of a financial liability incurred through the Alcon distribution
5
Taxes on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item
based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related
restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements
in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax eect. Due to these factors and the diering eective tax
rates in the various jurisdictions, the tax on the total adjustments of USD 7.6 billion to arrive at the core results before tax amounts to USD 1.2 billion. The average tax rate on the
adjustments is 15.7% since the full year core tax charge of 16.3% has been applied to the pre-tax income of the period.
6
Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.

Item 5. Operating and Financial Review andProspects
69
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions unless indicated otherwise) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
Operating income
Income before taxes
–
Income taxes
–
–
Net income
Basic EPS (USD)
The following are adjustments to arrive at core gross profit
Cost of goods sold –
–
The following are adjustments to arrive at core operating income
Selling, general and administration –
–
Research and development –
–
Other income
–
–
–
Other expense –
–
The following are adjustments to arrive at core income before taxes
Income from associated companies
–
Other financial income and expense –
–
–
1
Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets;
research and development includes the amortization of acquired rights for technologies; income from associated companies includes USD 210 million for the Novartis share of the
estimated Roche core items
2
Impairments: cost of goods sold and research and development include impairment charges related to intangible assets; other income and other expense include reversals of
impairment charges and impairment charges related to property, plant and equipment
3
Acquisition or divestment of businesses and related items, including restructuring and integration charges: other income includes adjustments to portfolio transformation and Alcon
spin-o accruals; other income and other expense include transitional service-fee income and expenses related to the Alcon distribution; other expense also includes adjustments
to provisions; income from associated companies includes the gain related to the divestment of our investment in Roche; other financial income and expense includes other
financial gains related to the divestment of our investment in Roche
4
Other items: cost of goods sold, research and development, other income and other expense include net restructuring and other charges related to the Group-wide rationalization
of manufacturing sites; cost of goods sold, selling, general and administration, other income and other expense include other restructuring income and charges and related items;
cost of goods sold, research and development, other income and other expense also include adjustments to contingent consideration; selling, general and administration, research
and development, other income and other expense include adjustments to provisions; other income and other expense also include gains and losses from the divestment of
products and financial assets and fair value adjustments on financial assets, adjustments to environmental provisions and legal-related items; other financial income and expense
includes a charge related to the monetary loss due to hyperinflation in Argentina and Venezuela and a revaluation impact of a financial liability incurred through the Alcon
distribution
5
Taxes on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item
based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related
restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements
in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax eect. Due to these factors and the diering eective tax
rates in the various jurisdictions, the tax on the total adjustments of USD 9.4 billion to arrive at the core results before tax amounts to USD 516 million. Excluding the gain on the
divestment of our investment in Roche, the tax on the total adjustments of USD 5.2 billion to arrive at the core results before tax amounts to USD 516 million and the average tax
rate on the adjustments was 10.0%.
6
Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
Item 5. Operating and Financial Review andProspects
70
2022 and 2021 reconciliation from IFRS results to core results – Innovative Medicines
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
–
Operating income
The following are adjustments to arrive at core gross profit
Other revenues
–
Cost of goods sold –
–
The following are adjustments to arrive at core operating income
Selling, general and administration –
–
Research and development –
–
–
Other income
–
–
Other expense –
–
1
Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets;
research and development includes the amortization of acquired rights for technologies
2
Impairments: cost of goods sold, research and development and other expense include impairment charges related to intangible assets; other income and other expense include
net impairment charges related to property, plant and equipment
3
Acquisition or divestment of businesses and related items, including restructuring and integration charges: other expense includes stamp duties related to an acquisition and
transitional service fee charges related to divestments
4
Other items: other revenues includes a net income from an outlicensing agreement; cost of goods sold, selling, general and administration, research and development, other
income and other expense include restructuring income and charges related to the initiative to implement a new streamlined organizational model, the Group-wide rationalization of
manufacturing sites and other net restructuring charges and related items; cost of goods sold and research and development also include contingent consideration adjustments
and adjustments to provisions and related items; other income and other expense include fair value adjustments and divestment gains and losses on financial assets and
legal-related items; other income also includes gains from the divestment of products and property, curtailment gains and an adjustment to an environmental provision; other
expense includes a reversal of an accrual and other costs and items
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
Operating income
–
The following are adjustments to arrive at core gross profit
Cost of goods sold –
–
The following are adjustments to arrive at core operating income
Selling, general and administration –
–
Research and development –
–
Other income
–
–
–
Other expense –
–
1
Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets;
research and development includes the amortization of acquired rights for technologies
2
Impairments: research and development includes impairment charges related to intangible assets; other income and other expense include reversals of impairment charges and
impairment charges related to property, plant and equipment
3
Acquisition or divestment of businesses and related items, including restructuring and integration charges: other income and other expense include transitional service fee income
and expenses related to the Alcon distribution
4
Other items: cost of goods sold, research and development, other income and other expense include net restructuring and other charges related to the Group-wide rationalization
of manufacturing sites; cost of goods sold, selling, general and administration, other income and other expense include other restructuring income and charges and related items;
cost of goods sold, research and development and other expense include adjustments to contingent consideration; selling, general and administration, research and development
and other expense include adjustments to provisions; other income and other expense include gains and losses from the divestment of products and financial assets and fair value
adjustments on financial assets; other expense also includes legal-related items and adjustments to environmental provisions

Item 5. Operating and Financial Review andProspects
71
2022 and 2021 reconciliation from IFRS to core results – Sandoz
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
Operating income
The following are adjustments to arrive at core gross profit
Cost of goods sold –
–
The following are adjustments to arrive at core operating income
Selling, general and administration –
–
Research and development –
–
Other income
–
–
Other expense –
–
1
Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets
2
Impairments: cost of goods sold and research and development include impairment charges related to intangible assets; other income includes a reversal of an impairment charge
related to property, plant and equipment
3
Other items: cost of goods sold, selling, general and administration, research and development, other income and other expense include charges related to the Sandoz strategic
review, the Group-wide rationalization of manufacturing sites and other net restructuring charges and related items; other expense also includes legal-related items; cost of goods
sold and selling, general and administration include adjustments to provisions and related items
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
Operating income
The following are adjustments to arrive at core gross profit
Cost of goods sold –
–
The following are adjustments to arrive at core operating income
Research and development –
–
–
Other income
–
–
Other expense –
–
1
Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets
2
Impairments: cost of goods sold and research and development include impairment charges related to intangible assets; other income and other expense include reversals of
impairment charges and impairment charges related to property, plant and equipment
3
Other items: cost of goods sold, other income and other expense include net restructuring and other charges related to the Group-wide rationalization of manufacturing sites and
other restructuring income and charges and related items; research and development includes adjustments to provisions; other income includes net gains from the divestment of a
product; other income and other expense include legal-related items
Item 5. Operating and Financial Review andProspects
72
2022 and 2021 reconciliation from IFRS results to core results – Corporate
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
Operating loss –
–
–
The following are adjustments to arrive at core operating loss
Selling, general and administration –
–
Other income
–
–
Other expense –
–
1
Impairments: other expense includes impairment charges related to intangible assets
2
Acquisition or divestment of businesses and related items, including restructuring and integration charges: other income includes adjustments to provisions and transitional service
fee income related to divestments
3
Other items: selling, general and administration, other income and other expense include restructuring income and charges related to the initiative to implement a new streamlined
organizational model, the Sandoz strategic review and other net restructuring charges and related items; other income and other expense also include fair value adjustments and
divestment gains and losses on financial assets; other income also includes a curtailment gain
Acquisition or
Amortization
divestment of
of intangible
businesses and
Other
(USD millions) IFRS results
assets
Impairments
related items
items
Core results
Gross profit
Operating loss –
–
–
The following are adjustments to arrive at core operating loss
Other income
–
–
Other expense –
–
1
Acquisition or divestment of businesses and related items, including restructuring and integration charges: other income includes adjustments to portfolio transformation and Alcon
spin-o accruals; other income and other expense include transitional service fee income and expenses related to the Alcon distribution; other expense also includes adjustments
to provisions
2
Other items: other income includes an adjustment to a contingent consideration receivable; other income and other expense include fair value adjustments and divestment gains
and losses on financial assets, adjustments to environmental provisions and restructuring income and charges and related items

Item 5. Operating and Financial Review andProspects
73
Reconciliation of 2021 IFRS results and non-IFRS measures core results and free cash flow to exclude the
impacts of the 2021 divestment of our Roche investment
To enhance investor understanding of the Group’s performance in comparison with the prior year, we presented
the 2021 IFRS results and non-IFRS measures core results and free cash flow excluding the impacts related to our
Roche investment, due to its divestment in the fourth quarter of 2021.
The following tables provide a reconciliation of our 2021 published IFRS results and non-IFRS measures core results
and free cash flow to the 2021 results, excluding the impacts related to our Roche investment, due to its divest-
ment.
2021
Results
Our Roche
excluding
investment
Gain on
impacts
impacts
divestment
from the
excluding
of our
divestment
Results as
the divestment
investment
of our Roche
(USD millions unless indicated otherwise) published
gain
in Roche
investment
Operating income
Income from associated companies
–
–
–
Interest expense and other financial
income and expense –
–
–
Income before tax
–
–
Income taxes –
–
Net income
–
–
Basic earnings per share (USD)
–
–
Eective tax rate
Core operating income
Core income from associated companies
–
–
Core interest expense and core other
financial income and expense –
–
Core income before tax
–
Core income taxes –
–
Core net income
–
Core basic earnings per share (USD)
–
Core eective tax rate
Free cash flow
–
1
Eective tax rate is calculated as Income taxes divided by Income before tax.
2
Core eective tax rate is calculated as Core income taxes divided by Core income before tax.
3
The free cash flow impact represents the dividend received in Q1 2021 from Roche in relation to the distribution of its 2020 net income.
Item 5. Operating and Financial Review andProspects
74
2021
Dividends
received from
Roche in
Free cash
relation to
flow excluding
the distribution
dividends
Free cash flow
of its
received
(USD millions) as published
net income
from Roche
Operating income
Adjustments for non-cash items
Operating income adjusted for non-cash items
Dividends received from associated companies and others
–
Interest and other financial payments, net –
–
Income taxes paid –
–
Other operating cash flow items, net –
–
Net cash flows from operating activities
–
Net purchases of property, plant and equipment, intangible assets, financial assets and
other non-current assets –
–
Free cash flow
–
1
In 2021, the dividend received from Roche in relation to the distribution of its 2020 net income was received in Q1 2021.
The following table provides a summary of the percentage point impact from excluding the eect of the divestment
of our investment in Roche (in the fourth quarter of 2021) on the USD and constant currencies % change on key
Group figures.
In USD In constant currencies
change
change
excluding
excluding
impacts
impacts
from the
from the
divestment
Percentage
divestment
Percentage
change
of our Roche
point
change
of our Roche
point
as published
investment
impact
as published
investment
impact
Net income –
–
–
–
–
–
Basic earnings per share (USD) –
–
–
–
–
–
Free cash flow –
–
–
Core net income –
–
–
Core basic earnings per share (USD) –
–
–
5.B Liquidity and capital resources
The following tables summarize the Group’s cash flows and net debt:
(USD millions)
Net cash flows from operating activities
Net cash flows from investing activities
Net cash flows used in financing activities –
–
Eect of exchange rate changes on cash and cash equivalents –
–
Net change in cash and cash equivalents –
Change in marketable securities, commodities, time deposits and derivative financial instruments –
Change in current and non-current financial debts and derivative financial instruments
Change in net debt –
Net debt at January –
–
Net debt at December –
–

Item 5. Operating and Financial Review andProspects
75
Cash flow
Financial year 2022 compared with 2021
Net cash flows from operating activities amounted to
USD 14.2 billion, compared with USD 15.1 billion in 2021.
This decrease was mainly due to unfavorable changes
in working capital and lower dividends from associated
companies (2021 included the USD 0.5 billion dividends
received from our investment in Roche, which was
divested in the fourth quarter of 2021), partly oset by
lower income taxes paid and favorable hedging results.
Net cash inflows from investing activities amounted
to USD 1.5 billion, compared with USD 4.2 billion in 2021.
The current year cash inflows were driven by net pro-
ceeds of USD 4.7 billion from the sale of marketable
securities, commodities and time deposits; USD 0.5 bil-
lion from the sale of intangible assets, financial assets
and property, plant and equipment. These cash inflows
were partly oset by cash outflows of USD 1.5 billion for
purchases of intangible assets; USD 1.2 billion for pur-
chases of property, plant and equipment; USD 0.1 billion
for purchases of financial assets; and USD 0.9 billion for
acquisitions and divestments of businesses, net (primar-
ily the acquisition of Gyroscope Therapeutics Holdings
plc for USD 0.8 billion).
In 2021, net cash inflows from investing activities of
USD 4.2 billion were driven by proceeds of USD 20.7 bil-
lion from the divestment of our investment in Roche; USD
2.3 billion from the sale of marketable securities, com-
modities and time deposits; and USD 1.4 billion from the
sale of intangible assets, financial assets and property,
plant and equipment. These cash inflows were partly o-
set by USD 16.4 billion cash outflows for purchases of
marketable securities and time deposits, mainly due to
the investment of a portion of the proceeds from the
divestment of our investment in Roche; USD 1.6 billion
for purchases of intangible assets (including the upfront
payment to in-license tislelizumab from an aliate of Bei-
Gene, Ltd); USD 1.4 billion for purchases of property,
plant and equipment; USD 0.6 billion for acquisitions and
divestments of businesses, net (including the acquisition
of GSK’s cephalosporin antibiotics business for USD 351
million); and USD 0.2 billion for purchases of financial
assets.
Net cash outflows used in financing activities
amounted to USD 20.6 billion, compared with USD 16.3
billion in 2021.
The current year cash outflows were mainly driven
by USD 10.6 billion for net treasury share transactions;
USD 7.5 billion for the dividend payment; USD 2.5 billion
in aggregate for the repayment of two US dollar bonds;
and USD 0.3 billion payments of lease liabilities. These
cash outflows were partly oset by cash inflows of USD
0.3 billion from the net increase in current financial debts.
In 2021, net cash outflows used in financing activities
of USD 16.3 billion were driven by USD 7.4 billion for the
dividend payment; USD 3.0 billion for net treasury share
transactions; USD 3.5 billion net decrease in current
financial debts; and USD 2.2 billion for the repayment of
two bonds denominated in euro (notional amount of EUR
1.25 billion and of EUR 0.6 billion) at maturity. Payments
of lease liabilities and other financing cash flows resulted
in a net cash outflow of USD 0.2 billion.
Free cash flow
Free cash flow is a non-IFRS measure, see “—Item 5.A Operating results—Non-IFRS measures as defined by
Novartis—Free cash flow” for further information.
The following table is a reconciliation of the three major categories of the IFRS consolidated statements of cash
flows to free cash flow:
2022 2021
IFRS
Free
IFRS
Free
(USD millions) cash flow
Adjustments
cash flow
cash flow
Adjustments
cash flow
Net cash flows from operating activities
Net cash flows from/(used in) investing activities
–
–
–
–
Net cash flows used in financing activities
–
–
Free cash flow
1
Excluded from the free cash flow are cash flows from investing activities associated with acquisitions and divestments of businesses and of interest in associated companies,
purchases and sales of marketable securities, commodities and time deposits.
2
Net cash flows used in financing activities are excluded from the free cash flow.

Item 5. Operating and Financial Review andProspects
76
The following table is a summary of the free cash flow:
(USD millions)
Operating income
Adjustments for non-cash items
Depreciation, amortization and impairments
Change in provisions and other non-current liabilities
Other
Operating income adjusted for non-cash items
Dividends received from associated companies and others
Interest and other financial receipts
Interest and other financial payments –
–
Income taxes paid –
–
Payments out of provisions and other net cash movements in non-current liabilities –
–
Change in inventories and trade receivables less trade payables –
–
Change in other net current assets and other operating cash flow items
Net cash flows from operating activities
Purchases of property, plant and equipment –
–
Proceeds from sale of property, plant and equipment
Purchases of intangible assets –
–
Proceeds from sale of intangible assets
Purchases of financial assets –
–
Proceeds from sale of financial assets
Purchases of other non-current assets –
–
Proceeds from sale of other non-current assets
Free cash flow
Financial year 2022 compared with 2021
Free cash flow amounted to USD 11.9 billion (–10% USD), compared with USD 13.3 billion in 2021, mainly due to a
decrease in net cash flows from operating activities and lower divestment proceeds, partly oset by lower pur-
chases of property, plant and equipment.

Item 5. Operating and Financial Review andProspects
77
Condensed consolidated balance sheets
(USD millions) Dec ,
Dec ,
Assets
Property, plant and equipment
Right-of-use assets
Goodwill
Intangible assets other than goodwill
Investments in associated companies
Deferred tax assets
Financial assets and other non-current assets
Total non-current assets
Inventories
Trade receivables
Other current assets and income tax receivables
Marketable securities, commodities, time deposits and derivative financial instruments
Cash and cash equivalents
Total current assets
Total assets
Equity and liabilities
Total equity
Liabilities
Financial debts
Lease liabilities
Deferred tax liabilities
Provisions and other non-current liabilities
Total non-current liabilities
Trade payables
Financial debts and derivative financial instruments
Lease liabilities
Provisions and other current liabilities and current income tax liabilities
Total current liabilities
Total liabilities
Total equity and liabilities
Assets
Total non-current assets of USD 80.5 billion at Decem-
ber 31, 2022, decreased by USD 5.5 billion compared to
December 31, 2021.
Intangible assets other than goodwill decreased by
USD 2.5 billion as additions (including the acquisition of
Gyroscope Therapeutics Holdings plc) were more than
oset by amortization, impairments and unfavorable cur-
rency translation adjustments.
Goodwill decreased by USD 0.3 billion, mainly due to
unfavorable currency translation adjustments.
Property, plant and equipment decreased by USD 0.8
billion, as net additions were more than oset by depre-
ciation, unfavorable currency translation adjustments
and impairments.
Financial and other non-current assets decreased by
USD 1.7 billion, driven by the decrease of the prepaid
post-employment benefit plans of USD 0.9 billion, result-
ing mainly from the pension accounting eects from
increases in actuarial discount rates and of USD 0.6 bil-
lion from fair value losses on listed equity and fund invest-
ments.
Right-of-use assets, investments in associated com-
panies and deferred tax assets were broadly in line with
December 31, 2021.
Total current assets of USD 36.9 billion at December
31, 2022, decreased by USD 8.8 billion compared to
December 31, 2021.
Cash and cash equivalents decreased by USD 4.9
billion, mainly due to the dividend payment, the purchase
of treasury shares and net repayments of financial debt,
partly oset by the cash generated from operating activ-
ities and from investing activities, which includes the net
proceeds from the sales of marketable securities, com-
modities and time deposits.
Marketable securities, commodities, time deposits
and derivative financial instruments decreased by USD
4.5 billion mainly driven by the net sales of marketable
securities, commodities and time deposits.
Inventories increased by USD 0.5 billion and trade
receivables and other current assets and income tax
receivables were broadly in line with December 31, 2021.
We consider our provisions for doubtful trade receiv-
ables to be adequate. We particularly monitor the level
of trade receivables in countries deemed to have an

Item 5. Operating and Financial Review andProspects
78
elevated credit risk. We consider macroeconomic envi-
ronment, historical experience, country and political risk,
in addition to other relevant information when assessing
risk. These risk factors are monitored regularly to deter-
mine any adjustments in risk classification. The majority
of the past due trade receivables from elevated credit
risk countries are due from local governments or from
government-funded entities. Deteriorating credit and
economic conditions as well as other factors in these
elevated credit risk countries have resulted in, and may
continue to result in, an increase in the average length
of time that it takes to collect these trade receivables
and may require the Group to re-evaluate the expected
credit loss amount of these trade receivables in future
periods. At December 31, 2022, amounts past due for
more than one year were not significant in elevated credit
risk countries.
For a table showing an overview of the aging analy-
sis of total trade receivables and the total amount of the
provision for doubtful trade receivables as of December
31, 2022, and 2021, see “Item 18. Financial Statements—
Note 15. Trade receivables.”
There is also a risk that certain countries could
devalue their currency. Currency exposures are
described in more detail in “—Eects of currency fluctu-
ations.”
Liabilities
Total non-current liabilities of USD 29.4 billion decreased
by USD 4.4 billion compared to December 31, 2021.
Non-current financial debts decreased by USD 2.7
billion, mainly due to the reclassification of USD 2.3 bil-
lion from non-current to current financial debts of two
EUR denominated bonds with notional amounts of EUR
750 million and EUR 1.25 billion maturing in 2023 and
favorable currency translation adjustments of USD 0.4
billion.
Provisions and other non-current liabilities decreased
by USD 1.3 billion, mainly driven by decreases in accrued
liabilities for employee benefits of USD 1.2 billion (primar-
ily due to a decrease in accrued liabilities for defined
benefit pension plans of USD 0.9 billion, resulting from
the pension accounting eects from increases in actu-
arial discount rates), and in contingent consideration of
USD 0.3 billion, a reclassification of non-current legal
matters provisions to current portion of USD 0.2 billion,
partly oset by the increase in other non-current liabili-
ties of USD 0.4 billion.
Deferred tax liabilities decreased by USD 0.4 billion
and non-current lease liabilities were broadly in line with
December 31, 2021.
Total current liabilities of USD 28.7 billion decreased
by USD 1.6 billion compared to December 31, 2021.
Provisions and other current liabilities and current
income tax liabilities decreased by USD 0.8 billion, mainly
driven by the decrease in the commitment for repurchase
of own shares liability of USD 2.8 billion, partly oset by
increases in restructuring provisions of USD 0.8 billion
(primarily due to the initiative announced in April 2022,
to implement a new streamlined organizational model),
in provisions for legal matters of USD 0.5 billion, includ-
ing a USD 0.2 billion reclassification from non-current
provisions for legal matters, and in provisions for reve-
nue deductions of USD 0.3 billion.
Current financial debts and derivative financial instru-
ments decreased by USD 0.4 billion, mainly due to the
repayment of two US dollar bonds of USD 1.0 billion and
USD 1.5 billion, the closure during the third quarter of
2022 of the interest-bearing accounts of employees pay-
able on demand, which amounted to USD 1.8 billion at
December 31, 2021, and favorable currency translation
adjustments, partly oset by the reclassification from
non-current to current financial debts of USD 2.3 billion
and an increase of USD 1.9 billion in commercial paper.
Trade payables decreased by USD 0.4 billion and cur-
rent lease liabilities were broadly in line with December
31, 2021.
In our key countries, Switzerland and the United
States, assessments have been agreed by the tax author-
ities up to 2017 in Switzerland and up to 2014 in the United
States, with the exception of one open United States
position related to the 2007 tax filing. Uncertainties also
exist on the application of a taxing right based on a Ger-
man non-resident tax regulation for specific revenues
derived from German registered intellectual property
rights.
Novartis believes that its total provisions are ade-
quate based upon currently available information. How-
ever, given the inherent diculties in estimating liabilities
in this area, Novartis may incur additional costs beyond
the amounts provided. Management believes that such
additional amounts, if any, would not be material to the
Group’s financial condition but could be material to the
results of operations or cash flows in a given period.
Equity
The Group`s equity decreased by USD 8.4 billion to USD
59.4 billion at December 31, 2022, compared to Decem-
ber 31, 2021.
This decrease was mainly due to the cash-dividend
payment of USD 7.5 billion, purchase of treasury shares
of USD 10.9 billion, unfavorable currency translation dif-
ferences of USD 0.5 billion and fair value adjustments on
equity securities of USD 0.4 billion. This was partially o-
set by the net income of USD 7.0 billion, decrease of the
treasury share repurchase obligation of USD 2.8 billion,
and equity-based compensation of USD 0.9 billion.

Item 5. Operating and Financial Review andProspects
79
Summary of equity movements attributable to Novartis AG shareholders
Number of outstanding shares Equity attributable to
(in millions) Novartis AG shareholders
USD millions
USD millions
Balance at beginning of year
Shares acquired to be canceled –
–
–
–
Other share purchases –
–
–
–
Exercise of options and employee transactions
Equity-based compensation
Shares delivered to Alcon employees as a result of the Alcon spin-o
Taxes on treasury share transactions
Decrease/(increase) of treasury share repurchase
obligation under a share buyback trading plan
–
Transaction costs, net of taxes
Dividends
–
–
Net income of the year attributable to shareholders of Novartis AG
Other comprehensive income attributable to shareholders of Novartis AG
–
–
Impact of change in ownership of consolidated entities
–
Other movements
Balance at end of year
1
Impact of hyperinflationary economies (see “Item 18. Financial Statements—Note 1. Significant accounting policies”).
In 2022, Novartis repurchased a total of 126.2 million
shares for USD 10.8 billion on the SIX Swiss Exchange
second trading line, including 115.3 million shares (USD
9.9 billion) under the up-to USD 15 billion share buyback
announced in December 2021 and 10.9 million shares
(USD 0.9 billion) to mitigate dilution related to participa-
tion plans of associates. In addition, 1.4 million shares
(USD 0.1 billion) were repurchased from associates. In
the same period, 12.3 million shares (for an equity value
of USD 0.9 billion) were delivered as a result of option
exercises and share deliveries related to participation
plans of associates. Consequently, the total number of
shares outstanding decreased by 115.3 million versus
December 31, 2021. These treasury share transactions
resulted in a decrease in equity of USD 10.0 billion and
a net cash outflow of USD 10.6 billion.
In 2021, Novartis repurchased a total of 30.7 million
shares for USD 2.8 billion on the SIX Swiss Exchange
second trading line, including 19.6 million shares (USD
1.8 billion) under the up-to USD 2.5 billion share buyback
announced in November 2020, 8.6 million shares (USD
0.8 billion) to mitigate dilution related to participation
plans of associates and 2.5 million shares (USD 0.2
billion) under the up-to USD 15 billion share buyback
announced in December 2021. In addition, 1.5 million
shares (USD 0.1 billion) were repurchased from associ-
ates. In the same period, 10.3 million shares (for an equity
value of USD 0.8 billion) were delivered as a result of
options exercised and share deliveries related to partic-
ipation plans of associates. Consequently, the total num-
ber of shares outstanding decreased by 21.9 million ver-
sus December 31, 2020. These treasury share
transactions resulted in a decrease in equity of USD 2.1
billion and a net cash outflow of USD 3.0 billion.
Treasury shares
At December 31, 2022, our holding of treasury shares
amounted to 284.1 million shares, or approximately 12%
of the total number of issued shares. Approximately 99.0
million treasury shares were held in entities that restrict
their availability for use.
At December 31, 2021, our holding of treasury shares
amounted to 199.5 million shares, or approximately 8%
of the total number of issued shares. Approximately
102.5 million treasury shares were held in entities that
restrict their availability for use.

Item 5. Operating and Financial Review andProspects
80
Eects of currency fluctuations
We transact our business in many currencies other than the US dollar, our reporting currency.
The following table provides an overview of net sales and operating expenses based on IFRS values for 2022 and
2021, for currencies most important to the Group:
2022 2021
Operating
Operating
Net sales
expenses
Net sales
expenses
Currency
US dollar (USD)
Euro (EUR)
Swiss franc (CHF)
Chinese yuan (CNY)
Japanese yen (JPY)
Canadian dollar (CAD)
British pound (GBP)
Russian ruble (RUB)
Brazilian real (BRL)
Australian dollar (AUD)
Other currencies
1
Operating expenses include cost of goods sold; selling, general and administration; research and development; other income and other expense.
We prepare our consolidated financial statements in US
dollars. As a result, fluctuations in the exchange rates
between the US dollar and other currencies can have a
significant eect on both the Group’s results of opera-
tions as well as the reported value of our assets, liabili-
ties and cash flows. This in turn may significantly aect
reported earnings (both positively and negatively) and
the comparability of period-to-period results of opera-
tions.
For purposes of our consolidated balance sheets, we
translate assets and liabilities denominated in other cur-
rencies into US dollars at the prevailing market exchange
rates as of the relevant balance sheet date. For purposes
of the Group’s consolidated income and cash flow state-
ments, revenue, expense and cash flow items in local
currencies are translated into US dollars at average
exchange rates prevailing during the relevant period. As
a result, even if the amounts or values of these items
remain unchanged in the respective local currency,
changes in exchange rates have an impact on the
amounts or values of these items in our consolidated
financial statements.
Because our expenditure in Swiss francs is signifi-
cantly higher than our revenue in Swiss francs, volatility
in the value of the Swiss franc can have a significant
impact on the reported value of our earnings, assets and
liabilities, and the timing and extent of such volatility can
be dicult to predict.
The Group manages its global currency exposure by
engaging in hedging transactions where management
deems appropriate, after taking into account the natural
hedging aorded by our global business activity. In 2022
and 2021, we entered into various contracts that change
in value with movements in foreign exchange rates, to
preserve the value of assets, commitments and expected
transactions. We use forward contracts and foreign cur-
rency options to hedge. For more information on how
these transactions aect our consolidated financial
statements and on how foreign exchange rate exposure
is managed, see “Item 18. Financial Statements—Note 1.
Significant accounting policies,” “Item 18. Financial State-
ments—Note 5. Interest expense and other financial
income and expense,” “Item 18. Financial Statements—
Note 15. Trade receivables,” “Item 18. Financial State-
ments—Note 28. Commitments and contingent liabilities”
and “Item 18. Financial Statements—Note 29. Financial
instruments – additional disclosures.”

Item 5. Operating and Financial Review andProspects
81
The following table sets forth the foreign exchange rates of the US dollar against key currencies used for foreign
currency translation when preparing the Group’s consolidated financial statements:
Average for year Year-end
USD per unit
Change in
Change in
Australian dollar (AUD)
–
–
Brazilian real (BRL)
Canadian dollar (CAD)
–
–
Swiss franc (CHF)
–
–
Chinese yuan (CNY)
–
–
Euro (EUR)
–
–
British pound (GBP)
–
–
Japanese yen (JPY ())
–
–
Russian ruble (RUB ())
The following table provides a summary of the currency impact on key Group figures due to their conversion into
US dollars, the Group’s reporting currency. For additional information on the constant currency calculation (“cc”),
see “—Item 5.A Operating results—Non-IFRS measures as defined by Novartis—Constant currencies”.
Currency impact on key figures
Change in
Percentage
Change in
Percentage
Change in
constant
point currency
Change in
constant
point currency
USD
currencies
impact
USD
currencies
impact
Total Group
Net sales to third parties –
–
Operating income –
–
–
Net income –
–
–
Basic earnings per share (USD) –
–
–
Core operating income
–
Core net income –
–
Core basic earnings per share (USD) –
–
Innovative Medicines
Net sales to third parties –
–
Operating income –
–
–
Core operating income
–
Sandoz
Net sales to third parties –
–
–
Operating income –
–
–
Core operating income –
–
–
–
–
Corporate
Operating loss –
–
nm
nm
nm
Core operating loss
–
–
–
nm = not meaningful
For additional information on the eects of currency fluctuations, see “Item 18. Financial Statements—Note 29.
Financial instruments – additional disclosures.”

Item 5. Operating and Financial Review andProspects
82
Group liquidity, financial debts and net debt
The following table shows Group liquidity, financial debts and net debt:
(USD millions)
Non-current financial debts –
–
Current financial debts and derivative financial instruments –
–
Total financial debts –
–
Less liquidity
Cash and cash equivalents
Marketable securities, commodities, time deposits and
derivative financial instruments
Total liquidity
Net debt at December
–
–
1
For further information about the net debt measure, which is a non-IFRS measure, see “—Item 5.A Operating results—Non-IFRS measures as defined by Novartis—Net debt.”
Financial year 2022
Group net debt at December 31, 2022, increased to
USD7.2 billion, compared with USD0.9 billion at Decem-
ber 31, 2021.
Total financial debts amounted to USD 26.2 billion at
December 31, 2022, compared with USD 29.2 billion at
December 31, 2021. Non-current financial debts
decreased by USD 2.7 billion, mainly due to the reclas-
sification of USD 2.3 billion from non-current to current
financial debts of two EUR denominated bonds with
notional amounts of EUR 750 million and EUR 1.25 bil-
lion maturing in 2023 and favorable foreign currency
translation adjustments of USD 0.4 billion.
Current financial debts and derivative financial instru-
ments decreased by USD 0.4 billion, mainly due to the
repayment of two US dollar bonds of USD 1.0 billion and
USD 1.5 billion, the closure during the third quarter of
2022 of the interest-bearing accounts of employees pay-
able on demand, which amounted to USD 1.8 billion at
December 31, 2021, and favorable currency translation
adjustments, partly oset by the reclassification from
non-current to current financial debts of USD 2.3 billion
and an increase of USD 1.9 billion in commercial paper.
Novartis has two US commercial paper programs
under which it can issue up to USD 9.0 billion in the
aggregate of unsecured commercial paper notes.
Novartis also has a Japanese commercial paper program
under which it can issue up to JPY 150 billion (approxi-
mately USD 1.1 billion) of unsecured commercial paper
notes. Commercial paper notes totaling USD 2.8 billion
under these three programs were outstanding as per
December 31, 2022 (2021: USD 0.9 billion).
Novartis also has a committed credit facility of
USD6.0 billion, which was extended in 2022. This credit
facility is provided by a syndicate of banks and is intended
to be used as a backstop for the US commercial paper
programs. The extended facility matures in September
2025 and was undrawn as per December 31, 2022, and
December 31, 2021.
Total liquidity decreased to USD 18.9 billion com-
pared with USD 28.3 billion at December 31, 2021.
As of year-end 2022, Moody’s Investors Service rated
the Company A1 for long-term maturities and P-1 for
short-term maturities and S&P Global Ratings rated the
company AA- for long-term maturities and A-1+ for short-
term maturities.
For the tables showing the maturity schedule of our
current financial assets, current and non-current finan-
cial debts and net debt at December 31, 2022 and
December 31, 2021, see “Item 18. Financial Statements—
Note 29. Financial instruments – Additional disclosures—
Nature and extent of risks arising from financial instru-
ments—Liquidity risk.”
For a description of risks and restrictions on the abil-
ity of subsidiaries to transfer funds to the Company via
cash dividends, loan or advances, please see “—Liquid-
ity/short-term funding” and “Item 18. Financial State-
ments—Note 29. Financial instruments – Additional dis-
closures—Nature and extent of risks arising from
financial instruments.”
Information regarding the Company’s material com-
mitments for capital expenditures as of the end of 2022
and 2021 and an indication of the general purpose of
such commitments and the anticipated sources of funds
needed to fulfill such commitments are provided in “—
Material short- and long-term cash requirements.”

Item 5. Operating and Financial Review andProspects
83
Liquidity and financial debt by currency
The following table provides a breakdown of liquidity and financial debt by currency as of December 31:
Financial
Financial
Liquidity
Liquidity
debt in
debt in
in
in
USD
CHF
EUR
JPY
Other
1
Liquidity includes cash and cash equivalents and marketable securities, including debt securities, commodities and time deposits.
2
Financial debt includes non-current and current financial debt.
Bonds
In April 2022, a 5-year USD denominated bond of USD
1.0 billion with a coupon of 2.40% was repaid, in advance
of its maturity date at no additional cost.
In September 2022, a 10-year USD denominated
bond of USD 1.5 billion with a coupon of 2.40% was
repaid at maturity.
In March 2021, a 4-year EUR denominated bond of
EUR 1.25 billion with a coupon of 0.00% was repaid at
maturity.
In November 2021, a 7-year EUR denominated bond
of EUR 0.6 billion with a coupon of 0.75% was repaid at
maturity.
Liquidity/short-term funding
The Group’s liquidity amounted to USD 18.9 billion at
December 31, 2022, compared with USD 28.3 billion at
December 31, 2021. Total non-current and current finan-
cial debts, including derivatives, amounted to USD 26.2
billion at December 31, 2022, compared with USD 29.2
billion at December 31, 2021.
The debt/equity ratio increased to 0.44:1 at Decem-
ber 31, 2022, compared with 0.43:1 at December 31,
2021. The net debt increased to USD 7.2 billion at
December 31, 2022, compared with USD 0.9 billion at
December 31, 2021.
We continuously track our liquidity position and
asset/liability profile. This involves modeling cash flow
maturity profiles based on both historical experiences
and contractual expectations to project our liquidity
requirements. We seek to preserve prudent liquidity and
funding capabilities. We are confident that we have suf-
ficient liquidity to support our normal business activities
for the foreseeable future.
Certain countries have legal or economic restrictions
on the ability of subsidiaries to transfer funds to the
Group in the form of cash dividends, loans or advances,
but these restrictions do not have an impact on the abil-
ity of the Group to meet its cash obligations.
We are not aware of any significant demands to
change the level of liquidity needed to support our nor-
mal business activities. We make use of various borrow-
ing facilities provided by several financial institutions. We
also successfully issued various bonds in previous years
and raised funds through our commercial paper pro-
grams.
The maturity schedule of our net debt can be found
in “Item 18. Financial Statements—Note 29. Financial
instruments – Additional disclosures—Nature and extent
of risks arising from financial instruments—Liquidity risk.”

Item 5. Operating and Financial Review andProspects
84
Material short- and long-term cash requirements
The following table summarizes the Group’s material short- and long-term cash requirements:
Payments due by period
Less than
After
(USD millions) Total
year
– years
– years
years
Non-current financial debt, including current portion
Interest on non-current financial debt, including current portion
Lease liabilities, non-current and current portion
Interest on lease liabilities, non-current and current portion
Commitments for leases not yet commenced
Unfunded pensions and other post-employment benefit plans
Research and development potential milestone commitments
Contingent consideration liabilities
Property, plant and equipment purchase commitments
Total contractual cash obligations
The Group intends to fund the research and develop-
ment; property, plant and equipment; intangible asset
purchase commitments with internally generated
resources, and the acquisition of business commitment
through available cash and short- and long-term borrow-
ings.
For other contingent liabilities, see “Item 8. Financial
Information—Item 8.A Consolidated statements and
other financial information,” “Item 18. Financial State
-
ments—Note 10. Right-of-use assets and lease liabilities,”
“Item 18. Financial Statements—Note 20. Provisions and
other non-current liabilities,” and “Item 18. Financial
Statements—Note 28. Commitments and contingent lia-
bilities.”

Item 5. Operating and Financial Review andProspects
85
5.C Research and development, patents and licenses
Our research and development spending totaled
USD10.0 billion and USD9.5 billion (Core research and
development USD9.1 billion and USD9.0 billion) for the
years 2022 and 2021, respectively.
Each of our divisions has its own research and devel-
opment and patents. Our divisions have numerous prod-
ucts in various stages of development. For further infor-
mation on these policies and these products in
development, see “Item 4. Information on the Company—
Item 4.B Business overview.”
As described in the risk factors section and else-
where in this Annual Report, our drug development
eorts are subject to the risks and uncertainties inher-
ent in any new drug development program. Due to the
risks and uncertainties involved in progressing through
preclinical development and clinical trials, and the time
and cost involved in obtaining regulatory approvals,
among other factors, we cannot reasonably estimate the
timing, completion dates and costs, or range of costs, of
our drug development programs, or of the development
of any particular development compound (see “Item 3.
Key Information—Item 3.D Risk factors”). In addition, for
a description of the research and development process
for the development of new drugs and our other prod-
ucts, and the regulatory process for their approval, see
“Item 4. Information on the Company—Item 4.B Business
overview.”
5.D Trend information
Please see “—Item 5.A Operating results”, “—Item 5.B
Liquidity and capital resources” and “Item 4. Information
on the Company—Item 4.B Business overview” for trend
information.
5.E Critical accounting estimates
Our consolidated financial statements are prepared in
accordance with International Financial Reporting Stan-
dards (IFRS) as issued by the International Accounting
Standards Board (IASB). The preparation of financial
statements requires management to make certain esti-
mates and assumptions, either at the balance sheet date
or during the year, which aect the reported amounts of
revenues, expenses, assets, liabilities and contingent
amounts. Our significant accounting policies that are set
out in “Item 18. Financial Statements—Note 1. Significant
accounting policies” include a description of the esti-
mates, assumptions and judgments applied in the prepa-
ration of the consolidated financial statements of the
Group.
Given the uncertainties inherent in our business activ-
ities, we must make certain estimates and assumptions
that require dicult, subjective and complex judgments.
Because of uncertainties inherent in such judgments,
actual outcomes and results may dier from our assump-
tions and estimates, which could materially aect the
Group’s consolidated financial statements. Application
of the following accounting policies requires certain
assumptions and estimates that have the potential for
the most significant impact on our consolidated financial
statements.
Management believes that the estimation uncertain-
ties described below did not have or are not reasonably
likely to have a material impact on the Group’s financial
condition but could be material to the results of opera-
tions or cash flows in a given period.
Deductions from revenues
As is typical in the pharmaceutical industry, the consid-
eration we receive in exchange for goods and services
maybe fixed or variable. The most common elements of
variable consideration are primarily composed of rebates
and discounts granted to wholesalers, retailers, govern-
ment agencies, government supported healthcare sys-
tems, private health systems, pharmacy benefit manag-
ers, managed healthcare organizations and other
customers. Variable consideration is recognized when it
is highly probable that a significant reversal will not occur.
These elements of variable consideration represent esti-
mates of the related obligations, requiring the use of judg-
ment when estimating the eect of these considerations
for a reporting period.
The following summarizes the nature of some of
these deductions and how the deduction is estimated.
After recording these, net sales represent our best esti-
mate of the cash that we expect to ultimately collect. The
US market has the most complex arrangements related
to revenue deductions.
United States-specific healthcare plans and
program rebates
The United States Medicaid Drug Rebate Program is
administered by state governments, using state and fed-
eral funds to provide assistance to certain vulnerable
and needy individuals and families. Calculating the
rebates to be paid related to this program involves use
of estimates and interpreting relevant regulations, which
are subject to challenge or change in interpretative

Item 5. Operating and Financial Review andProspects
86
guidance by government authorities. Provisions for esti-
mated Medicaid rebates are calculated using a combi-
nation of historical experience, product and population
growth, product pricing, and the mix of contracts and
specific terms in the individual state agreements.
The United States Federal Medicare Program, which
funds healthcare benefits to individuals aged 65 and
older, and to people with certain disabilities, provides
prescription drug benefits under the Part D section of
the program. This benefit is provided and administered
through private prescription drug plans. Calculating the
rebates to be paid related to this program involves use
of estimates and interpreting relevant regulations, which
are subject to challenge or change in interpretative guid-
ance by government authorities. Provisions for estimated
Medicare Part D rebates are calculated based on the
terms of individual plan agreements, product sales and
population growth, product pricing, including inflation
impacts, and the mix of contracts.
We oer rebates to key managed healthcare and pri-
vate plans in an eort to ensure patient access to our
products and to sustain and increase the market share
of our products. These programs provide a rebate after
the plans have demonstrated they have met all terms and
conditions set forth in their contract with us.
These rebates and discounts, applied using provision
rates, are estimated based on the specific terms in the
individual states and plans agreements, historical expe-
rience, product pricing and projected product growth
rates, as appropriate to the individual rebate and dis
-
count arrangements, and are recorded as a deduction
from revenue at the time the related revenues are
recorded.
These provisions are adjusted based on established
processes and experiences from filing data with individ-
ual states and plans. There is often a time lag between
recording of revenue deductions and the final account-
ing for them.
Non-United States-specific healthcare plans and
program rebates
In certain countries other than the US, we provide rebates
to governments and other entities. These rebates are
often mandated by laws or government regulations.
These rebates, applied using provision rates, are esti-
mated based on government regulations, laws and terms
of individual rebate arrangements, historical experience
and other relevant factors, and are recorded as a deduc-
tion from revenue at the time the related revenue is
recorded. These estimates are adjusted periodically to
reflect actual experience. There is often a time lag
between the recording of revenue deductions and the
final accounting for them.
Innovative pay-for-performance arrangements
We enter into innovative pay-for-performance arrange-
ments (i.e. outcome based arrangements) with certain
healthcare providers and governments. Under these
agreements, we may be required to make refunds, defer
a portion of the sales price until anticipated treatment
outcomes meet predefined targets, or to provide addi-
tional medicines free of charge if anticipated treatment
outcomes do not meet predefined targets.
The impact of potential refunds or a deferral of a por-
tion of the sales price are estimated and recorded as a
deduction from revenue at the time the related sales are
recorded. The impact of the future delivery of additional
medicines at no cost is estimated and recorded as a con-
tract liability at the time the related revenues are recorded.
Estimates are based on historical experience and clini-
cal data available for the product, as well as specific
terms of the individual agreements. In cases where his-
torical experience and clinical data are not sucient for
a reliable estimation of the outcome, revenue recogni-
tion is deferred until the uncertainty is resolved, until such
history is available or the period of the refund right has
expired.
These provisions for revenue deductions are adjusted
periodically based on established processes and actual
experience, including the products’ actual outcomes
achieved compared with the anticipated predefined tar-
gets.
There is often a time lag between recording of the
revenue deductions and the final accounting for them.
Non-healthcare plans and program rebates, returns
and other deductions
We oer rebates to purchasing organizations and other
direct and indirect customers to sustain and increase
market share and to ensure patient access to our prod-
ucts. Since rebates are contractually agreed upon, the
related provisions are estimated based on the terms of
the individual agreements, historical experience and pro-
jected product sales growth rates.
Chargebacks occur where our subsidiaries have
arrangements with indirect customers to sell products
at prices that are lower than the price charged to whole-
salers. A chargeback represents the dierence between
the invoice price to the wholesaler and the indirect cus-
tomer’s contract price. We account for chargebacks by
reducing revenue by the estimate of chargebacks attrib-
utable to a sales transaction. Provisions for estimated
chargebacks are calculated using a combination of fac-
tors, such as historical experience, product growth rates,
product pricing, level of inventory in the distribution chan-
nel, and the terms of individual agreements.
When we sell a product providing a customer the right
to return it, we record a provision for estimated sales
returns based on our sales return policy and historical
return rates. Other factors considered include actual
product recalls, expected marketplace changes, the
remaining shelf life of the product, and the expected
entry of generic products. In 2021, sales returns amounted
to approximately 1% of gross product sales. If sucient
experience is not available, sales are only recorded
based on evidence of product consumption or when the
right of return has expired.
We enter into distribution service agreements with
major wholesalers, which provide a financial disincentive
for the wholesalers to purchase product quantities in
excess of current customer demand. Where possible,
we adjust shipping patterns for our products to maintain
wholesalers’ inventory levels consistent with underlying
patient demand.
We oer cash discounts to customers to encourage
prompt payment. Cash discounts are estimated and

Item 5. Operating and Financial Review andProspects
87
provisioned at the time of revenue recognition and are
deducted from revenue.
Following a decrease in the price of a product, we
generally grant customers a “shelf stock adjustment” for
their existing inventory for the relevant product. Shelf
stock adjustments are generally granted to customers,
primarily of the Sandoz Division, to cover the inventory
held by them at the time a price decline becomes eec-
tive. Revenue deduction provisions for shelf stock adjust-
ments are recorded when the price decline is anticipated,
based on the impact of the price decline on the custom-
er’s estimated inventory levels.
Other sales discounts, such as consumer coupons,
vouchers and copay discount cards, are oered in some
markets. The estimated amounts of these discounts are
recorded at the time of sale or when the coupons are
issued, and are estimated utilizing historical experience
and the specific terms for each program.
In addition, we oer global patient assistance pro-
grams.
We adjust provisions for revenue deductions period-
ically to reflect actual experience. To evaluate the ade-
quacy of provision balances, we use internal and exter-
nal estimates of the inventory in transit, the level of
inventory in the distribution and retail channels, actual
claims data received, and the time lag for processing
rebate claims. External data sources include reports
from wholesalers and third-party market data purchased
by Novartis.
For the table showing the worldwide extent of our reve-
nue deductions provisions and related payment experi-
ences for the Group see “Item 18. Financial Statements—
Note 22. Provisions and other current liabilities.”
Impairment of goodwill, intangible
assets and property, plant and
equipment
We review intangible assets and property, plant and
equipment for impairment whenever events or changes
in circumstance indicate that the asset’s balance sheet
carrying amount may not be recoverable. Goodwill and
other intangible assets that are not yet amortized, are
reviewed for impairment at least annually.
An asset is considered impaired when its balance
sheet carrying amount exceeds its estimated recover-
able amount, which is defined as the higher of its fair value
less costs of disposal and its value in use. Usually, Novartis
applies the fair value less costs of disposal method for
its impairment assessment. In most cases, no directly
observable market inputs are available to measure the
fair value less costs of disposal. Therefore, an estimate
is derived indirectly and is based on net present value
techniques utilizing post-tax cash flows and discount
rates. In the limited cases where the value in use method
would be applied, net present value techniques would be
applied using pre-tax cash flows and discount rates.
Fair value less costs of disposal reflects estimates of
assumptions that market participants would be expected
to use when pricing the asset or cash generating units
(CGUs), and for this purpose, management considers
the range of economic conditions that are expected to
exist over the remaining useful life of the asset.
The estimates used in calculating the net present val-
ues are highly sensitive and depend on assumptions spe-
cific to the nature of the Group’s activities as indicated
in “Item 18. Financial Statements—Note 1. Significant
accounting policies.” Due to these factors, actual cash
flows and values could vary significantly from forecasted
future cash flows and related values derived using dis-
counting techniques.
The recoverable amount of the grouping of cash-gen-
erating units to which goodwill is allocated is based on
fair value less costs of disposal. The valuations are
derived from applying discounted future cash flows
based on key assumptions, including the terminal growth
rate and discount rate. For additional information on
impairment charges recognized and reversed by divi
-
sions, see “Item 18. Financial Statements—Note 1. Sig-
nificant accounting policies—Impairment of goodwill and
intangible assets” and “Item 18. Financial Statements—
Note 11. Goodwill and intangible assets.”
Goodwill and other intangible assets represent a sig-
nificant part of our consolidated balance sheet, primar-
ily due to acquisitions. Although no significant additional
impairments are currently anticipated based on our
impairment assessment and review of reasonable pos-
sible changes in key assumptions to the respective
impairment assessment, future impairment evaluation
could lead to material impairment charges in the future.
For more information, see “Item 18. Financial State-
ments—Note 11. Goodwill and intangible assets.”
For net impairment charges for property, plant and
equipment see “Item 18. Financial Statements—Note 9.
Property, plant and equipment.”
Retirement and other post-
employment benefit plans
We sponsor pension and other post-employment bene-
fit plans in various forms that cover a significant portion
of our current and former employees. For post-employ-
ment plans with defined benefit obligations, we are
required to make significant assumptions and estimates
about future events in calculating the expense and the
present value of the liability related to these plans. These
include assumptions about the interest rates we apply
to estimate future defined benefit obligations and net
periodic pension expense, as well as rates of future pen-
sion increases. In addition, our actuarial consultants pro-
vide our management with historical statistical informa-
tion, such as withdrawal and mortality rates in connection
with these estimates.
Assumptions and estimates used by the Group may
dier materially from the actual results we experience
due to changing market and economic conditions, higher
or lower withdrawal rates, and longer or shorter life spans
of participants, among other factors.
Depending on events, such dierences could have a
material eect on our total equity.
For more information on obligations under retirement
and other post-employment benefit plans and underly-
ing actuarial assumptions, see “Item 18. Financial

Item 5. Operating and Financial Review andProspects
88
Statements—Note 25. Post-employment benefits for
employees.”
Income taxes
We prepare and file our tax returns based on an inter-
pretation of tax laws and regulations, and we record esti-
mates based on these judgments and interpretations.
Our tax returns are subject to examination by the com-
petent taxing authorities, which may result in an assess-
ment being made, requiring payments of additional tax,
interest or penalties. Since Novartis uses its intellectual
property globally to deliver goods and services, the
transfer prices within the Group as well as arrangements
between subsidiaries to finance research and develop-
ment and other activities may be challenged by the
national tax authorities in any of the jurisdictions in which
Novartis operates. Therefore, inherent uncertainties
exist in our estimates of our tax positions, but we believe
that our estimated amounts for current and deferred tax
assets or liabilities, including any amounts related to any
uncertain tax positions, are appropriate based on cur-
rently known facts and circumstances. Uncertain
(income) tax positions are periodically (re)assessed by
the Company based on management’s best judgment
given any changes in the facts, circumstances and infor-
mation available and applicable tax laws. When it is prob-
able that the tax authorities will not accept the position
taken, the Group recognizes income tax liabilities based
on the most likely amount of the liability (recovery) or
weighted average of various possible outcomes to reflect
the eect of the uncertainty in determining the related
taxable profit (tax loss), tax bases, unused tax losses,
unused tax credits or tax rates, to the extent that a reli-
able estimate can be made.
For more information, see “Item 18. Financial State-
ments—Note 6. Income taxes” and “Item 18. Financial
Statements—Note 12. Deferred tax assets and liabilities.”
Provisions and contingent liabilities
A number of Group companies are involved in various
government investigations and legal proceedings (intel-
lectual property, sales and marketing practices, product
liability, commercial, employment and wrongful dis-
charge, environmental claims, etc.) arising out of the nor
-
mal conduct of their businesses.
We record provisions for legal proceedings when it
is probable that a liability has been incurred and the
amount can be reliably estimated. These provisions are
adjusted periodically as assessments change or addi-
tional information becomes available. For significant
product liability cases, the provision is actuarially deter-
mined based on factors such as past experience, amount
and number of claims reported, and estimates of claims
incurred but not yet reported.
Provisions are recorded for environmental remedia-
tion costs when expenditure on remedial work is proba-
ble and the cost can be reliably estimated.
Novartis believes that its total provisions are ade-
quate based upon currently available information. How-
ever, given the inherent diculties in estimating liabilities
in this area, Novartis may incur additional costs beyond
the amounts provided. Management believes that such
additional amounts, if any, would not be material to the
Group’s financial condition but could be material to the
results of operations or cash flows in a given period.
For more information, see “Item 18. Financial State-
ments—Note 20. Provisions and other non-current liabil-
ities” and “Item 18. Financial Statements—Note 28. Com-
mitments and contingent liabilities.”

Item 6. Directors, Senior Management and Employees
89
Item 6. Directors, Senior Management and
Employees
6.A Directors and senior management
The information set forth under “Item 6. Directors, Senior
Management and Employees—Item 6.C Board prac-
tices—Corporate governance—Board of Directors” and
“Item 6. Directors, Senior Management and Employees—
Item 6.C Board practices—Corporate governance—
Executive Committee” is incorporated by reference.

Item 6. Directors, Senior Management and Employees
90
6.B Compensation
Dear shareholder,
I am pleased to share with you the Novartis Compensa-
tion Report for 2022.
We believe that our compensation system supports
our strategy and motivates our executives to deliver sus-
tainable growth, successful outcomes on our financial
and strategic targets, and value creation for our share-
holders. Over the course of 2022, we engaged with
shareholders and proxy advisors to share how our com-
pensation system is aligned with short and long-term
performance, and to secure their continued support for
our compensation system design. Based on the feed-
back from these interactions and the positive response
to our 2021 Compensation Report, which received a
90.7% vote in favor, we will retain the current design of
our executive compensation system, with small enhance-
ments as explained later in this letter.
2022 performance highlights
2022 was a year of solid financial performance, with
growth in constant currencies (cc) across sales, core
profits and core margins. Sales growth drivers were
Entresto (USD 4.6 billion), Kesimpta (USD 1.1 billion),
Kisqali (USD 1.2 billion), Cosentyx (USD 4.8 billion), along
with the Pluvicto launch. Our six in-market growth driv-
ers with multi-billion sales potential (Cosentyx, Entresto,
Zolgensma, Kisqali, Kesimpta and Leqvio) grew 26% (cc)
in 2022, and now represent 32% of total Innovative
Medicines sales, up from 26% in 2021. Overall sales were
broadly in line with target.
In April 2022, we announced the introduction of a new
organizational model designed to support the company’s
innovation, growth, and productivity ambitions as a
focused medicines company. The restructuring will sim-
plify the organization and our processes, and is expected
to deliver USD 1.5 billion in savings by 2024 with a pro-
portion of these savings already delivered in 2022,
enabling us to raise our long-term core operating income
margin guidance. These savings are expected to help us
progress towards our aspiration of achieving ~40%+ core
margin beyond 2027 and further invest in our pipeline, as
a pure-play medicines company (after the planned
Sandoz spin-o which is subject to approval of the
Novartis AG Board of Directors and shareholders). None-
theless, there was an immediate impact on Operating
Income as we incorporated related costs in the latter part
of the year, that, along with higher legal settlements and
unfavorable fair market value adjustments on financial
assets, impacted operating income growth.
In 2022, we continued to deliver high value medicines
to patients. We received 23 approvals in our key focus
markets US, EU, China and Japan, including US and EU
approvals for Pluvicto, a novel radioligand therapy for
advanced prostate cancer. We advanced our focused
pipeline of investigational medicines, with several import-
ant clinical data readouts paving the way for further
launches in 2023 and beyond, a significant one being
iptacopan, our investigational monotherapy in the treat-
ment of paroxysmal nocturnal hemoglobinuria (PNH).
However, we also had disappointments as some clinical
trials of experimental compounds did not meet their pri-
mary endpoints, including ACZ885 (canakinumab) in
lung cancer, and UNR844 in presbyopia.
We are proud that Novartis also continued to deliver
on its commitments to broaden access to medicines and
tackle major global health challenges. We pledged fur-
ther investment in research into malaria and neglected
tropical diseases, increased access to our innovative
medicines for low- and middle-income countries and
formed new collaborations with governments and other
partners to strengthen healthcare systems. More details
on our ESG eorts can be found in our Novartis in Soci-
ety Integrated Report 2022.
2022 CEO compensation
As a result of the above performance, the CEO was
awarded a 2022 Annual Incentive of 100%, having over-
all met the financial targets and strategic objectives set
at the beginning of the cycle. When determining perfor-
mance against the operating income metric, the Board
of Directors approved adjustments to exclude restruc-
turing costs arising from the implementation of the new
organizational model and costs related to the planned
Sandoz spin-o, which are investments in the future of
the company in terms of both sales and margin growth.
These adjustments ensured that the performance
assessment was consistent with the basis on which the
original targets were set.
The 2020-2022 Long-Term Performance Plan (LTPP)
vested at 57% of target. The LTPP outcome was heavily
aected by the relative Total Shareholder Return (rTSR)
performance over the period, as well as reduced sales
growth during 2020 and 2021, which was substantially
impacted by the COVID-19 pandemic. No adjustments
were made for the COVID-19 impact, or for any other
factors. (For more information, please see “—LTPP per-
formance outcomes”).
Despite a solid 2022 performance, as outlined above,
the CEO’s 2022 total realized compensation was CHF
8452176, a decrease of 24.7% compared with prior year,
driven mainly by the 2020-2022 LTPP outcome.
Changes to Executive Committee compensation
system and disclosures
During the year, we reviewed our Executive Committee
compensation system, with the aim of simplification and
increased transparency of our performance assessment
measures and strengthening our focus on key strategic
priorities, while also considering developments in com-
pensation best practices.
Eective the 2022-2024 cycle of the LTPP, we
strengthened the assessment of research and early
development performance under the Innovation metrics,

Item 6. Directors, Senior Management and Employees
91
to ensure that targets are focused more directly on activ-
ities that create long-term value, and are measurable
over a three-year performance period. For the innova-
tion performance measure, the Science & Technology
Committee sets targets that take into account the
expected Net Present Value (eNPV) of programs transi-
tioning to late-stage clinical development rather than the
previous approach to set targets related to early-stage
milestones.
Eective from performance year 2023, we will remove
“Share of Peers” as a financial performance measure for
the Annual Incentive plan, to simplify the metrics and
focus on targets that provide greater transparency. The
weighting of the three remaining financial measures,
Group Net Sales, Group Operating Income and Group
Free Cash Flow, will be 40%, 30% and 30%, respectively.
In addition, we will fold division specific financial targets,
where applicable, into individual strategic objectives
(40% weighting) of the related Executive Committee
member. All Executive Committee members will be eval-
uated, with a 60% weighting, against the performance
of Group financial measures mentioned above.
During the year, we announced our intention to sep-
arate our Sandoz generics and biosimilars Division into
a new publicly traded standalone company, by way of a
100% spin-o, subject to approval of the Novartis AG
Board of Directors and shareholders. Based on the
planned completion of the spin-o in 2023, the Compen-
sation Committee made some initial decisions on the
2023 compensation elements related to the spin-o.
Finally, the 2023 Compensation Report will also
include additional disclosures following the reform of
Swiss corporate law that came into eect on January 1,
2023. For more information, please see “—2023 Execu-
tive Compensation Changes”.
Inflation and cost-of-living impact on broader
employee group
The Board and the Executive Committee are mindful of
the cost-of-living challenges that are impacting many of
our associates in dierent markets. The Executive
Committee has considered these as part of its pay deci-
sions and outcomes, and where appropriate, it has initi-
ated local level initiatives to support associates.
In most countries, our 2023 salary budgets are higher
than in previous years, reflecting the overall higher mar-
ket forecasts driven by inflation. In some of our larger
markets we are making a one-time payment to certain
employee populations. Where legally possible, we have
tried to target these one-time payments to our lower paid
employees, who are most impacted. We will continue to
monitor our compensation against the Living Wage, and
regularly monitor and adjust wages in hyperinflation mar-
kets to support our local associates.
These actions reflect our commitment to pay mar-
ket-competitive and sustainable salaries, rather than to
fully match the current volatile inflation environment.
2023 base salary increases for ECN members,
including the CEO, are made in line with policy, and no
ECN member will receive any inflation related one-time
payments.
2023 Annual General Meeting (AGM)
At the 2023 AGM, shareholders will be asked to vote on
both the maximum aggregate amount of compensation
for the Board of Directors from the 2023 AGM to the 2024
AGM, and the maximum aggregate amount of compen-
sation for the Executive Committee for the financial year
2024. Furthermore, we will request an advisory vote on
this Compensation Report.
We welcome your feedback, which is invaluable in
driving improvements in our compensation system and
practices. On behalf of the Compensation Committee, I
would like to thank you for your continued support and
trust.
Simon Moroney, D.Phil.
Chair of the Compensation Committee
Item 6. Directors, Senior Management and Employees
92
Compensationataglance
2022 outcomes
CEO pay for performance
CEO total realized compensation
The 2022 total realized compensation for the CEO was CHF 8 452 176. It includes payouts of the Annual Incentive
and LTPP based on actual performance assessed for the cycles concluding in 2022. More information on the
assessment of the CEO by the Board of Directors can be found in “—2022 CEO balanced scorecard” and “ —LTPP
performance outcomes”.
Board compensation
The total actual compensation earned by Board members in the 2022 financial year is shown in the table below.
CHF s total compensation
Board Chair
Other members of the Board
Total
1
Includes an amount of CHF 29250 for mandatory employer contributions for all Board members paid by Novartis to Swiss governmental social security systems. This amount is out
of total employer contributions of CHF 453083 and provides a right to the maximum future insured government pension benefit for the Board members.
Annual base salary
Pension and other benefits
2022 Annual Incentive
LTPP 2020–2022 cycle
Fixed pay
and benefits
(CHF 000s)
Variable pay:
Performance-related (CHF 000s)
Total realized compensation: CHF 8 452 176
1
The amounts shown represent the underlying share value of the total number of shares vested (including dividend equivalents of CHF 317 316) to the CEO for the 2020-2022 LTPP
performance cycle.
1 787 674
29% of total
2 684 3 307
1
32% of total 39% of total
200%
150%
100%
50%
0%
200%
150%
100%
50%
0%
% of target
% of target
Payout: 100% of target
Payout:
57% of target
Maximum
Target
Maximum
• Net sales CAGR
(43% of target)
• Core operating income CAGR
(93% of target)
• Innovation
(92% of target)
• Relative TSR
(0% of target)
2022 Annual Incentive
Long-Term Performance Plan (2020–2022 performance)
{
Annual base salary Pension and
other benefits
2023
Annual Incentive
Long-Term Incentive
awards cycle 2023-2025
LTPP
1
2023 fixed pay and benefits
Reflects responsibilities,
experience and skill sets
Cash
–
Provide retirement and
risk insurances (tailored
to local market practices/
regulations)
Country/individual-
specific and aligned with
other employees
–
Rewards performance
against short-term
financial and strategic
objectives, and Values and
Behaviors
50% cash
50% equity
2
deferred
for three years
3
Balanced scorecard
comprising:
• Financial measures
4
(60%)
• Strategic objectives
5
(40%)
Rewards long-term share-
holder value creation and
innovation in line with our
strategy
Equity, vesting following a
three-year performance
period
• Net sales
CAGR (25%)
• Core operating income
CAGR (25%)
• Innovation (25%)
• Relative TSR (25%)
Performance-related variable pay
Purpose
Form of payment
Performance measures
1
LTPP = Long-Term Performance Plan
2
Executive Committee members may elect to receive more of their Annual Incentive in equity instead of cash
3
The Annual Incentive deferred in equity is granted under the Deferred Share Bonus Plan (DSBP)
4
Financial Measures are Group Net Sales (40%), Group Operating Income (30%) and Group Free Cash Flow (30%)
5
Strategic objectives are aligned with our transformation to become a pure-play Innovative Medicines company: Strategy, Growth / Launches, Innovation, Operational excellence, Build trust with
society
Item 6. Directors, Senior Management and Employees
93
2023 compensation systems
An overview of the 2023 compensation systems for the Executive Committee and the Board of Directors is provided
below.
Executive Committee compensation system
Eective 2023, financial measures of the Annual Incentive plan comprise Group Net Sales (40%), Group Operat-
ing Income (30%) and Group Free Cash Flow (30%) for all Executive Committee members. Additionally, “Share of
Peers” will be removed from the financial measures.
Board compensation system
There are no changes to the Board compensation system for 2023.
AGM -
CHF s annual fee
Board Chair
Board membership
Vice-Chair
Lead Independent Director
Chair of the Audit and Compliance Committee
Chair of the Compensation Committee
Chair of the following committees:
• Governance, Sustainability and Nomination Committee
• Science & Technology Committee
• Risk Committee
Membership of the Audit and Compliance Committee
Membership of the following committees:
• Compensation Committee
• Governance, Sustainability and Nomination Committee
• Science & Technology Committee
• Risk Committee
Pay for
performance
• Variable compensation is tied directly to the
achievement of strategic Company targets
Shareholder
alignment
• Our incentives are significantly weighted
toward long-term equity-based plans
• Measures under the Long-Term Incentive
plans are calibrated to promote the creation
of shareholder value
• Executive Committee members are
expected to build and maintain substantial
shareholdings
Balanced
rewards
• Balanced set of measures to create
sustainable value
• Mix of targets based on financial metrics,
strategic objectives, and performance versus
our competitors
Business
ethics
• The Novartis Values and Behaviors are an
integral part of our compensation system
• They underpin the assessment of overall
performance for the Annual Incentive
Competitive
compensation
• Total compensation must be sufficient to
attract and retain key global talent
• Overarching emphasis on pay for
performance
GLOBAL HEALTHCARE PEER GROUP
AbbVie
Biogen
GlaxoSmithKline
Novo Nordisk
Roche
Amgen
Bristol-Myers Squibb
Gilead Sciences
Merck & Co.
Sanofi
AstraZeneca
Eli Lilly & Co.
Johnson & Johnson
Pfizer
Item 6. Directors, Senior Management and Employees
94
Executive Committee
compensationphilosophy and principles
Novartis compensation philosophy
Our compensation philosophy aims to ensure that we
attract and retain outstanding Executive Committee
members and reward them according to their success
in implementing the Company strategy, and their contri-
bution to Company performance and long-term value
creation. The main elements of our compensation phi-
losophy are set out in the table below.
Alignment with Company strategy
Executive compensation is strongly connected to busi-
ness strategy. In 2022, we refocused our strategy to
deliver high-value medicines that alleviate society’s
greatest disease burdens through technology leadership
in research and development, and novel access
approaches. Our strategy focuses on five core thera-
peutic areas with high unmet patient needs, two core
and three emerging technology platforms, and four pri-
ority geographies, which together account for the major-
ity of expected growth in global healthcare spending.
In line with this refocused strategy, we updated our
strategic priorities to target innovation power, sales
growth, delivering both margin and total shareholder
returns, and sector leadership in material ESG factors.
The Long-Term Incentive Plan was adapted, with greater
emphasis now on the delivery of high value programs in
our research and early development targets. The Annual
Incentive plan has been simplified eective 2023, with
three key financial metrics: Net Sales, weighted 40%;
Operating Income, weighted 30%; and Free Cash Flow,
weighted 30%.
Approach to market benchmarking
There continues to be significant competition for top
executive talent with deep expertise, the requisite com-
petencies and proven performance within the pharma-
ceutical and biotechnology industries. As such, external
peer compensation data is one of a number of key refer-
ence points considered by the Board of Directors and the
Compensation Committee when making decisions on
executive pay, so as to help ensure that the compensa-
tion system and compensation levels at Novartis remain
competitive. Novartis is committed to confirming bench-
marking practices, including the peer group, to sharehold-
ers on an annual basis.
The Compensation Committee believes in a rigorous
approach to peer group construction and maintenance.
Furthermore, it believes that using a consistent set of
peers that is similar in size and scope enables sharehold-
ers to evaluate the compensation year on year and make
pay-for-performance comparisons. In 2022, the Com-
pensation Committee decided to maintain the same pri-
mary peer group of 14 global healthcare companies,
as presented in the table below.
The companies in this peer group reflect our industry
and are similar to Novartis in terms of both size and scope
of operations. Although Novartis is headquartered in
Switzerland, more than a third of its sales come from the
US market, and the US remains a significant talent pool
for the recruitment of executives by the Company. It is
therefore critical that Novartis is able to attract and retain
key talent globally, especially from the US.
To ensure European and local practices were fully
taken into account, in 2022 the Compensation Commit-
tee also reviewed a cross-industry peer group of
Europe-headquartered multinational companies,
selected based on comparability to Novartis in terms of
industry, size, global scope of operations, and economic
influence. Based on this review, the Committee retained
the same group of European peers as in 2021: Anheus-
er-Busch InBev, AstraZeneca, Bayer, BMW, GlaxoSmith-
Kline, L’Oréal, Merck KGaA, Nestlé, Novo Nordisk, Reck-
itt Benckiser, Roche, Siemens, Sanofi, and Unilever.
ELEMENT OF COMPENSATION POLICY
Level The overall package should be market-competitive to enable the recruitment of global executive talent with
deep expertise and competencies.
Annual base salary The Compensation Committee may appoint individuals who are new to a role on an annual base salary
that is below the market level, with a view to increase this toward market level over a period of three to four
years as an individual develops in the role.
If the scope of an existing Executive Committee member’s role changes significantly during the year, the
Compensation Committee may make adjustments to the individual’s base salary (and/or incentives) in
consideration of the benchmark of the new role and the Executive Committee appointments compensation
policy.
This prudent approach ensures pay levels are merit-based, with increases dependent on strong
performance and proven ability in the role over a sustained period.
Incentives The compensation package will normally include the key compensation elements and incentive
opportunities in line with those offered to current Executive Committee members.
In exceptional circumstances, higher incentive opportunities than those offered to current Executive
Committee members may be provided at the Compensation Committee’s discretion.
Performance measures may include business-specific measures tailored to the specific role.
Pension and other benefits Newly appointed Executive Committee members are eligible for the local country pension plan and other
benefits in line with the wider employee group.
Buyouts The Compensation Committee seeks to balance the need to offer competitive compensation opportunities
to acquire the talent required by the business with the principle of maintaining a strong focus on pay for
performance.
As such, when an individual forfeits variable compensation as a result of an appointment at Novartis,
the Compensation Committee may offer replacement awards to compensate the commercial equivalent
value or fair value of payments and awards forfeited by the individual, in such form as the Compensation
Committee considers appropriate, taking into account relevant factors.
Relevant factors include the expected value of the forfeited award, the replacement vehicle (i.e., cash,
restricted share units, restricted shares or performance share units), whether the award is contingent on
meeting performance conditions or not, the timing of forfeiture (i.e., Novartis mirrors the blocking or vesting
period of the forfeited award) and the leaver conditions, in case the recruited individual leaves Novartis
prior to the end of the blocking or vesting period.
International mobility If individuals are required to relocate or be assigned away from their home location to take up their position,
relocation support may be provided in line with our global mobility policies (e.g., relocation support, tax
equalization). This includes ongoing US state income tax liabilities on behalf of US citizens locally employed
outside the US who have US workdays and therefore, US state taxable compensation that generates a US
state tax liability.
Item 6. Directors, Senior Management and Employees
95
Executive Committee appointments compensation policy
ELEMENT OF COMPENSATION POLICY
Annual Incentive –
cash element
Retirement, termination by the Company (for reasons other than performance or conduct), change of
control, disability, death, i.e., “good leavers”
Pro-rata Annual Incentive is paid to reflect the portion of the year the individual was employed.
Voluntary resignation or termination by the Company for misconduct or poor performance
Annual Incentive is fully forfeited.
Annual Incentive – mandatory
deferral into restricted shares/
restricted share units (RSUs)
Retirement, termination by the Company for reasons other than performance or conduct, and change
of control
Awards are released on the original blocking end date. There is no accelerated vesting. All awards are
subject to forfeiture in the event that a leaver joins a competitor company as defined in the applicable plan
rules, before the end of the three-year blocking date, starting from the date of grant.
Death or long-term disability
Accelerated vesting is applied.
Voluntary resignation or termination by the Company for misconduct or poor performance
Unvested restricted shares and restricted share units (RSUs) are forfeited.
Annual Incentive – voluntary
restricted shares/RSUs/American
Depository Receipts (ADRs)
(ADRs applicable for
US employees only)
Awards are not subject to forfeiture during the deferral period.
Long-Term Incentive – mandatory
performance share units (PSUs)
Retirement, termination by the Company for reasons other than performance or conduct, and change
of control
Awards vest on the regular vesting date, subject to performance, on a pro-rata basis for time spent with
the Company during the performance cycle. There is no accelerated vesting. All awards are subject to
forfeiture in the event that a leaver joins a competitor company as defined in the applicable plan rules, until
the vesting date.
Death or long-term disability
Accelerated vesting at target is applied.
Voluntary resignation or termination by the Company for misconduct or poor performance
All of the award is forfeited.
Item 6. Directors, Senior Management and Employees
96
Treatment of variable compensation for Executive Committee leavers
Malus and clawback
Any incentive compensation paid to Executive Commit-
tee members is subject to malus and clawback rules.
This means that the Board of Directors for the CEO, and
the Compensation Committee for the other Executive
Committee members, may decide – subject to applica-
ble law – to retain any unpaid or unvested incentive com-
pensation (malus), or to recover incentive compensation
that has been paid or vested in the past (clawback). This
applies in cases where the payout has resulted from a
violation of laws or conflicts with internal management
standards, including Company and accounting policies.
This principle applies to both the short-term Annual
Incentive and Long-Term Incentive (LTI) plans.
The Compensation Committee is assessing the
impact of the final clawback rule in the Federal Register
published by the US Securities and Exchange Commis-
sion in 2022, and any required changes to the policy will
be disclosed in the 2023 Compensation Report.
Objective setting
• The CEO proposes their targets to the
Board Chair; they are then reviewed and
approved by the Board of Directors,
based on input from the Compensation
Committee.
• For other Executive Committee
members, targets for their division
or unit are initially discussed with the
CEO and subsequently approved
by the Board of Directors and the
Compensation Committee.
Performance evaluation
• The CEO’s performance against
the individual balanced scorecard is
assessed by the Board of Directors.
• For Executive Committee members,
the CEO discusses each member’s
performance (assessed against his
or her individual balanced scorecard)
with the Board Chair before making
recommendations to the Board of
Directors for final determination.
• Periodic assessments, including at the
mid-year stage, ensure progress is
suitably tracked.
Compensation determination
• A recommendation for the CEO’s
variable pay is made by the
Compensation Committee to the Board
of Directors for final determination.
• For the Long-Term Incentive financial
measures’ payout schedules, a
formulaic approach applies, and the
Compensation Committee can also
exercise judgment to ensure there
is appropriate alignment between
payout levels and overall performance
achieved. The same principle of
discretion applies to the relative TSR
and innovation performance measures.
• The CEO’s recommendations for
other Executive Committee members
are considered and approved by the
Compensation Committee, after which
the Board of Directors is notified of the
outcomes.
Item 6. Directors, Senior Management and Employees
97
Executive Committee performancemanagement process
To foster a high-performance culture, the Company
applies a performance management process based on
quantitative and qualitative criteria. The CEO and the
other Executive Committee members are subject to a
formal three-step process: objective setting, perfor-
mance evaluation and compensation determination. This
process is explained in the chart below.
Performance targets are generally set before the
start of the relevant performance cycle. A rigorous
framework is in place for establishing targets to ensure
they are suitably robust and challenging, and align with
the strategic priorities of the Group.
The key factors taken into account when setting tar-
gets include:
• Internal and external market expectations
• Novartis strategic priorities
• Regulatory factors (e.g., new launches, patent expiries)
• Investment in capital expenditure
• Values and Behaviors
The targets are challenged at multiple stages before they
are ultimately approved by the Board of Directors. In line
with good governance practices, the Compensation
Committee works to set targets that are ambitious and
challenging but do not encourage undue risk-taking.
Following the end of the performance cycle, the Board
of Directors and the Compensation Committee consider
performance against the targets originally set. The CEO
and Executive Committee members are not present while
the Board of Directors and the Compensation Commit-
tee discuss their individual performance evaluations and
determine their individual compensation. Prior to deter-
mining the final outcome, related factors such as perfor-
mance relative to peers, wider market conditions, general
industry trends and good practice are used to inform the
overall performance assessment.
Annual base salary
Overview • The annual base salary is reviewed each year, taking into account: the individual’s role, performance and
experience; business performance and the external environment; increases across the Group; and market
movements.
2022 annual base salaries The 2022 annual base salaries were as follows:
• CEO (effective March 1, 2022): CHF 1 789 500.
• OTHER EXECUTIVE COMMITTEE MEMBERS (effective March 1, 2022): All other members of the
Executive Committee were awarded increases in line with the average of all Novartis employees, with the
exception of five individuals as disclosed in Item 6.B of the 2021 Annual Report.
Pension and other benefits
Overview • Pension and other benefits do not constitute a significant proportion of total compensation and are
provided to the Executive Committee on the same terms as all other employees based on local country
practices and regulations.
• The CEO and all other Swiss-based members of the Executive Committee are members of the Novartis
Swiss pension funds, which provide Company contributions on the base salary and Annual Incentive up to
the legal cap on the insured salary of CHF 860 400. No supplementary pension plans or savings plans are
provided. The CEO’s employer pension contributions represent 9.77% of his base salary.
• Globally the Company operates both defined benefit and defined contribution pension plans (see also Note
25 to the Group’s consolidated financial statements).
• Novartis may provide other benefits according to local market practice. These include Company car
provision, tax and financial planning, and insurance benefits.
• Executive Committee members who are required to relocate internationally may also receive additional
benefits (including tax equalization), in line with the Company’s global mobility policies.
Item 6. Directors, Senior Management and Employees
98
2022 Executive Committee compensation
PLAN OVERVIEW
Target Annual Incentive
On-target opportunities • CEO: 150% of annual base salary
• Other Executive Committee members: 80% to 120% of annual base salary
Performance measures • An Annual Incentive balanced scorecard containing:
• Financial performance measures (60% weighting) related to Group, division or business unit, where relevant
• Strategic objectives (40% weighting) are aligned with our transformation to become a pure-play
Innovative Medicines company: Strategy, Growth / Launches, Innovation, Operational excellence, Build
trust with society
• The balanced scorecard targets and achievements of the CEO are detailed on the next page.
• The balanced scorecards for other Executive Committee members include Group financial targets as well
as financial or other quantitative targets that relate to their division or business unit, if applicable.
• Values and Behaviors are a key component of the Annual Incentive and are embedded in our culture. As
such, members of the Executive Committee are expected to demonstrate these to the highest standards.
Target setting • Financial targets are set at the beginning of each financial year and align with the strategic plan proposed
by management to the Board of Directors for approval.
• The strategic objectives are aligned with the most important priorities in any performance year.
Payout ranges • The payout schedule for the Annual Incentive incorporates performance against financial and strategic
objectives. The payout range is 0% to 200% of on-target opportunity based on performance, as shown
below:
PERFORMANCE PAYOUT (% of on-target)
Outstanding 170% – 200%
Exceeds expectations 130% – 160%
Meets expectations 80% – 120%
Partially meets expectations 40% – 70%
Below expectations 0%
Payout formula
Payout vehicle • At the end of the performance period, 50% is paid in cash, and the remaining 50% is delivered in Novartis
restricted shares or RSUs, deferred for three years (see “—Executive Committee compensation system”).
• Executives may choose to receive all or part of the cash portion of their Annual Incentive in Novartis shares
or American Depositary Receipts (ADRs; US only) that will not be subject to forfeiture conditions. In the US,
awards may also be delivered in cash under the US-deferred compensation plan.
Dividend rights, voting rights
and settlement
• Novartis restricted shares and ADRs carry voting rights and dividends during the vesting period. RSUs are
of equivalent value but do not carry voting rights and dividends during the vesting period.
• Following the vesting period, settlement of RSUs is made in unrestricted Novartis shares or ADRs.
Annual base
salary
Target incentive
(% of base salary)
Target
Annual Incentive
x =
Annual base
salary
Target incentive
(% of base salary)
Payout factor (% of
target: 0%–200%)
Realized
Annual Incentive
x x =
Item 6. Directors, Senior Management and Employees
99
2022 Annual Incentive
CEO BALANCED SCORECARD
This section presents the balanced scorecard for the CEO. Balanced scorecard performance is measured in constant cur-
rencies (cc) to reflect operational performance that can be influenced. The Board of Directors uses a stringent process to set
ambitious financial targets to incentivize superior performance. In addition to the financial targets, the CEO also has ambitious
strategic objectives across key priority areas, including targets related to ESG matters.
Achievement versus
CEO achievements – 2022 Target target
Financial measures – 60% of total Annual Incentive, comprising:
Group net sales (cc) (30%) 54 360 million Met
Group operating income (cc) (30%)
|
11 630 million
|
M et *
Group free cash flow as a % of sales (cc) (20%)
|
24. 8 %
|
Bel ow
Share of peers for Novartis Group (20%) 7.3% Met
Overall assessment of Group financial targets in constant currencies Met
* The Board concluded that the achievement for Group operating income versus target was “Met” after approving adjustments mainly to exclude restructuring costs arising from the
implementation of the new organizational model announced to investors on April 4, 2022 (and were not available at the time of target setting in January 2022), and costs related to
the planned Sandoz spin-o, to transform Novartis into a focused medicines company.
Strategic objectives – 40% of total Annual Incentive, comprising:
Strategy (15%)
|
Met
In 2022, the CEO launched a new strategy and laid the foundation to improve our growth profile via a strong focus
on our five core therapeutic areas (cardiovascular, immunology, neuroscience, solid tumors, and hematology), two
established (chemistry and biotherapeutics) and three emerging (gene & cell therapy, radioligand therapy, and
xRNA) technology platforms, and four key geographies (China, Germany and Japan, and a particular priority in
the US market). This strategy will transform Novartis into a pure-play Innovative Medicines business, with multiple
in-market brands of multi-billion dollar peak sales potential, and prioritize our pipeline to focus on high-value assets
that address high disease burden and have substantial growth potential.
Sandoz separation analysis was completed with spin-off being the preferred separation path given potential future
value upside for shareholders. Substantial progress was also made on the preparation for the planned spin-off,
which is expected to take place in the second half of 2023.
Growth/Launches (15%)
|
Met
Recent launch products Pluvicto (USD 271 million), Kesimpta (USD 1.1 billion), and Scemblix (USD 149 million) achie-
ved higher than target sales. However, lower uptake for Leqvio resulted in sales behind target.
In-market growth drivers (including Cosentyx, Entresto, Zolgesma, Kisqali, Kesimpta, and Leqvio) delivered com-
bined sales of USD 13.2 billion, which was slightly behind target. This was largely due to the below target perfor-
mance of Cosentyx (total sales of USD 4.8 billion, impacted by US payer pressures, China business and Inflation
Reduction Act headwinds). This was partly offset by strong performance of Entresto (USD 4.6 billion) and Kisqali
(USD 1.2 billion).
Innovation (15%)
|
Met
In 2022, we received 23 approvals in our top four markets (US, EU, China and Japan). Major approvals included
Pluvicto (US, EU), Scemblix (EU), and further indication expansions for Kymriah and Cosentyx.
24 submissions were made across the top four markets. We advanced our focused pipeline of investigational
medicines, with several important clinical data readouts including Iptacopan for patients with paroxysmal nocturnal
hemoglobinuria (PNH), a rare and deadly blood disorder, and Pluvicto in earlier lines of prostate cancer. Cosentyx
was submitted to the US FDA for an additional indication, ahead of planned timelines.
Among our early-stage development activities, we secured ten proofs of concept (POCs) / proofs of mechanisms
(POMs). Additionally, we achieved First Patient First Visit in six pivotal trial-enabling studies against our Research
and Development target of five.
The year also experienced some disappointments, with important trials not meeting primary goals (such as cana-
kinumab for lung cancer and UNR844 in presbyopia).
Item 6. Directors, Senior Management and Employees
100
CEO BALANCED SCORECARD − CONTINUED
Operational excellence (15%)
|
Met
In April 2022, we introduced a new operating model to make our organization more agile and efficient in support of
our strategy. This simplified and leaner organization is expected to deliver identified cost savings of approximately
USD 1.5 billion by 2024, and help drive mid-term Innovative Medicines margin to the low 40s.
Financial performance for 2022 improved from prior year in constant currencies on core operating income and
core margin to USD 16.7 billion and 33.0% respectively.
The Operations unit, comprising our legacy Technical Operations unit and the legacy Customer and Technology
Solutions unit, achieved savings of USD 998 million against a combined target of USD 785 million. However, these
savings were partially offset by external headwinds, driven mainly by inflation, of approximately USD 350 million.
Build trust with society (40%)
|
Above
INNOVATION AND ACCESS
In 2022, we achieved a 26% increase in patient reach with our strategic innovative therapies, reaching 1.2 million
patients, compared with the previous year (0.95 million).
All our product launches in 2022 included a tiered pricing strategy based on national income level and value-based
pricing, in line with target.
With our commitment to diversity in clinical trials, 100% of our US Phase 3 studies evaluated Diversity & Inclusion
principles in feasibility planning, in line with our target.
Through our Novartis Global Health flagship programs, we reached 31 million patients in 2022, beating our Sustain-
ability-linked Bond target of 22.6 million patients by 2025.
In 2022, we renewed our commitment to the research and development of new medicines for malaria and neglec-
ted tropical diseases, pledging to invest USD 250mn over five years (2021-2025), and we advanced the clinical
development of next-generation malaria medicines.
PEOPLE AND CULTURE
We progressed towards a “Performance Culture” mindset with the implementation of a new “high support / high
challenge” approach.
We remain committed in our efforts to increase workforce diversity. The percentage of women in management
increased to 47%, slightly behind our target of 48%.
ENVIRONMENTAL SUSTAINABILITY
In 2022, we reduced our Scope 1 and 2 carbon emissions by 49%, our water consumption by 42%, and our waste
sent for disposal by 59%, compared with our 2016 baseline. This was broadly in line or ahead of our 50%, 41% and
50% targets, respectively. To advance on our Scope 3 emissions target, environmental sustainability criteria have
been integrated into supply contracts covering more than a third of our Scope 3 supplier emissions.
ETHICAL BUSINESS PRACTICES
We assessed 100% of our applicable policies & controls in the areas of Access to Medicine & Artificial Intelligence
and categorized them as either “aligned with Human Right standards” or “needs update with defined scope to meet
Human Rights standards”, in line with our target.
Overall assessment of strategic objectives Met
Overall assessment of CEO balanced scorecard Met
ANNUAL INCENTIVE PAYOUT
Payout The 2022 CEO performance showed solid financial results, including sales and operating income
performance at target and most strategic objectives were achieved or exceeded. The launch of a new
focused strategy transforming Novartis into a pure-play medicines company, performance of launch
products and preparation for planned Sandoz spin-off were key highlights. However, Free Cash Flow
performance was impacted by decrease in net cash flows from operating activities and lower divestment
proceeds. On balance, based on the overall assessment, the Board of Directors decided on an Annual
Incentive payout for the CEO amounting to CHF 2 684 321, which is 100% of target, within the range of
0–200%.
Item 6. Directors, Senior Management and Employees
101

OVERVIEW OF LONGTERM PERFORMANCE PLAN
Award vehicle Performance share units (PSUs) are granted at the beginning of the three-year performance cycle and vest
at the end of the cycle to the extent that performance conditions have been met. At the time of vesting, they
are converted into Novartis shares.
PSUs carry dividend equivalents that are paid in shares at the end of the cycle.
Grant formula At the start of the performance cycle, PSUs are granted under the Long-Term Incentive plan, as follows:
Target opportunity • CEO: 325% of annual base salary
• Other Executive Committee members: between 180% and 260% of annual base salary
Performance measures • Net sales CAGR (25%)
• Core operating income CAGR (25%)
• Innovation (25%)
• Relative TSR (25%)
Target setting Financial targets: Targets for net sales CAGR and core operating income CAGR are set based on the
strategic plan of the Company.
Innovation: Global Drug Development (GDD) targets are based on targeted filings communicated at the
start of each performance cycle, weighted 70%. The Science & Technology Committee determines the most
important Novartis Institutes for BioMedical Research (NIBR) milestones, weighted 30%. Effective the 2022-
2024 LTPP cycle, NIBR targets set by the Science & Technology Committee take into account the expected
Net Present Value (eNPV) of programs transitioning to late-stage clinical development.
Payout range Financial targets: When assessing performance, achievements for threshold, target and maximum payout are
defined for each metric, and a payout curve is applied to determine the corresponding payout between 0–200%
against target.
Innovation: At the end of the cycle, the Compensation Committee determines the payout factor in the range
of 0–150% based on the performance assessment made by the Science & Technology Committee. A payout
between 150–200% of target is only delivered for truly exceptional performance.
Relative TSR: Performance on TSR is assessed relative to a global healthcare peer group, as outlined below.
A three-month averaging method is used for both the start and the end of the performance cycle. Companies
are then ranked in order of highest to lowest TSR in USD.
The Compensation Committee may use its discretion on each metric, including deciding on the payout
within the ranges where appropriate. In doing so, it takes into consideration factors such as the underlying
assumptions of the targets set at the beginning of the cycle, overall economic conditions, currency
fluctuations and other unforeseeable situations.
Payout formula
Annual base
salary
Grant value
Target
incentive %
Share price
Grant value
Target number of
PSUs
x
/
Step 1
Step 2
=
=
Target number of
PSUs
Performance factor
Dividend
equivalents
Realized PSUsx + =
Global healthcare peer group
Abbvie
Biogen
GlaxoSmithKline
Novo Nordisk
Roche
Amgen
Bristol-Myers Squibb
Gilead Sciences
Merck & Co.
Sanofi
AstraZeneca
Eli Lilly & Co
Johnson & Johnson
Pfizer
Novartis position Payout range
in the peer group (% of target)
Position 1 – 2
Position 3 – 5
Position 6 – 8
Position 9 – 15
170% – 200%
130% – 160%
80% – 120%
0%
Item 6. Directors, Senior Management and Employees
102
Long-Term Performance Plan, 2020-2022 cycle
Item 6. Directors, Senior Management and Employees
103
LTPP performance outcomes
The charts below illustrate the performance of the 2020-2022 LTPP against target.
NET SALES CAGR
(25% weighting)
Vesting range 0–200% of target
8%
6%
4%
2%
0%
Maximum (200%): 8.7% (CAGR)
Net sales
growth payout
43% of target
Actual: 3.8% (CAGR)
CORE OPERATING INCOME COI CAGR
(25% weighting)
16%
12%
8%
4%
0%
Maximum (200%): 16.6% (CAGR)
COI growth payout
93% of target
Vesting range 0–200% of target
INNOVATION
(25% weighting)
The following developments were considered in our 2020-2022 LTPP
innovation performance:
• US and EU approvals for Pluvicto
• EU approval for Scemblix for adult patients with chronic myeloid leu-
kemia
• US approval for Kymriah in the treatment of adult patients with
relapsed or refractory follicular lymphoma
• Filing of Cosentyx for Hidradenitis suppurativa with both the US FDA
and the European Medicines Agency(EMA)
• Submission of Cosentyx for an additional indication ahead of planned
timelines
• Tislelizumab’s acceptance by the EMA for regulatory review in esoph-
ageal and lung cancers
• CANOPY trials, Ligelizumab PEARL studies in chronic spontaneous
urticaria (CSU), and Sabatolimab STIMULUS MDS-1 where import-
ant trial milestones were delayed/not submitted
• In NIBR, advancement of multiple development candidates including
two novel radioligand therapies
Based on input from the Science & Technology Committee, the Board
of Directors approved an innovation performance factor of 92% of tar-
get.
RELATIVE TOTAL SHAREHOLDER RETURN TSR
(25% weighting)
Novartis position Payout range
in the peer group (% of target)
Position 1 – 2
Position 3 – 5
Position 6 – 8
Position 9 – 15
170% – 200%
130% – 160%
80% – 120%
0%
Actual ranking
12
th
= 0% of target
Novartis achieved a net sales CAGR of 3.8% (in constant currencies –
cc) against the 5.7% target set at the beginning of the performance
cycle. The lower than target performance was mainly due to the nega-
tive and unexpected impact of COVID-19 in 2020 and 2021, the Beovu
safety update, and the slower uptake of Zolgensma.
Following the application of the payout curve, the net sales CAGR
(cc) achievement generates a payout factor of 43% (maximum 200%)
for this metric.
Novartis achieved a COI CAGR of 9.9% (cc) against the 10.6% target
set at the beginning of the performance cycle. This was mainly due to
lower than target Innovative Medicines sales over the three-year cycle,
which was partly oset by lower spend in selling, general and adminis-
trative expenses (SG&A). In 2022, the Company took organizational
transformative measures, delivering savings reflected in COI improve-
ment for the year.
Following the application of the payout curve, the COI CAGR (cc)
achievement generates a payout factor of 93% (maximum 200%) for
this metric.
Notes:
A minimum achievement of 6.6% CAGR was required to receive a payout under
this performance measure
Actual performance was adjusted for mergers and acquisitions as well as
business development and licensing projects not included in the target
Notes:
A minimum achievement of 3.7% CAGR was required to receive a payout under
this performance measure
TSR for the 2020-2022 cycle was 5.5%. As a result, Novartis ranked
No. 12 out of 15 healthcare companies (including Novartis). Considering
that the relative TSR rank is below median, there was a zero payout for
this metric.
LTPP PAYOUT
Net sales CAGR
43% x 25%
COI CAGR
93% x 25%
Innovation
92% x 25%
Relative TSR
0% x 25%
Final vesting
57% of target
+ + +
Overall, the Board of Directors approved a 2020-2022 LTPP payout at 57% of target, within the range of 0–200%. No adjustments, pandemic-
related or otherwise, were made in the evaluation of performance, despite the substantial shortfall in sales growth caused by Covid-19. This resulted
in an LTPP payout of CHF 3 307 422 for the CEO, including dividend equivalents of CHF 317 316.
Target: 5.7% (CAGR)
Actual: 9.9% (CAGR)
Target: 10.6% (CAGR)

Item 6. Directors, Senior Management and Employees
104
Compensation for joining and departing Executive Committee members
in2022
2022 Executive Committee member appointments
In 2022, four new appointments were made to the Executive Committee, which comprise an internal promotion and
three external appointments.
Victor Bulto was promoted internally as President, Innovative Medicines US, and joined the Executive Commit-
tee on May 1, 2022.
In line with our compensation policy, externally appointed Executive Committee members were granted buyout
awards to compensate for entitlements forfeited by them as a result of joining Novartis, as described in the table
below (see “—Executive Committee appointments compensation policy”). Further details on the vesting of the
awards below will be provided in relevant future compensation reports.
Name Date of appointment Currency Cash payments Equity awards Total value at grant
Shreeram Aradhye, May , CHF No cash buyout RSUs,
President, Global Drug vesting over the period -
Development and
Chief Medical Ocer
Aharon Gal, July , CHF RSUs,
Chief Strategy & Growth Ocer vesting over the period -
Fiona Marshall, November , USD to be paid RSUs and PSUs,
President, Novartis Institutes out in March vesting over the period -
for BioMedical Research
2022 Executive Committee member departures
In determining the compensation arrangements for departing Executive Committee members, the Compensation
Committee ensures that contractual entitlements are respected, and all payments are in line with our plan rules
and the Swiss Ordinance against Excessive Compensation in Listed Companies.
All Executive Committee members have a 12-month notice period during which they are entitled to their con-
tractual base salary, pension, Annual Incentive and other benefits. No new LTPP grants are made during the notice
period. In line with the new regulations arising from the reform of Swiss corporate law, any compensation payments
toward non-competition agreements from 2023 onwards, will not exceed the average annual compensation of the
previous three financial years.
Equity plan rules state that malus and clawback as well as non-compete restrictions will continue to apply. No
severance payments are made to departing Executive Committee members. Further details on the policy treatment
of variable compensation for departing Executive Committee members can be found in “—Treatment of variable
compensation for Executive Committee leavers.”
Former President of Novartis Oncology, Susanne Schaert, stepped down from her role following the Compa-
ny’s decision to integrate the Pharmaceuticals and Oncology business units and create separate US and Interna-
tional commercial organizations under the Innovative Medicines (IM) Division, and started her notice period on May
1, 2022.
Former Head of Customer & Technology Solutions (CTS), Robert Weltevreden, stepped down from his role fol-
lowing the Company’s decision to combine Novartis Technical Operations (NTO) and CTS into a new Operations
unit, and started his notice period on May 1, 2022.
Former Head of Global Drug Development and Chief Medical Ocer, John Tsai, stepped down from his role
eective May 15, 2022, and started his notice period on the same day.
Former President of the Novartis Institutes for BioMedical Research (NIBR), James Bradner, stepped down from
his role eective October 31, 2022, and started his notice period on November 1, 2022.
All four executives departed under good leaver conditions. Outstanding LTI grants will vest at the end of the rel-
evant performance cycles on a pro-rata basis, as per their contractual agreements and in line with the said plan
rules.
To avoid a conflict of interest, Richard Saynor, Chief Executive Ocer of Sandoz, stepped down from the Execu-
tive Committee with eect from October 25, 2022, following his appointment as CEO designate of the Sandoz
standalone company that is planned to be created in the second half of 2023. He will continue to report directly to
the CEO and to lead the Sandoz division.
Item 6. Directors, Senior Management and Employees
105
Realized compensation
To aid shareholders’ understanding of the link between pay and performance, the Compensation Committee dis-
closes the realized compensation for the CEO individually, and for the other members of the Executive Committee
on an aggregated basis. Disclosing realized compensation means that the Annual Incentive and the LTI are dis-
closed at the end of their respective performance cycles, reflecting actual payouts based on performance.
The total actual payout may vary year on year depending on multiple factors, including the composition of the
Executive Committee and the tenure of its members (as new members may not have a vested LTI), compensation
increases, payout of variable compensation based on actual performance, share price fluctuations of the LTI, and
dividend equivalents.
2022 realized compensation for the CEO and other Executive Committee members
The table below shows fixed and other compensation for the year, including the Annual Incentive for the 2022 per-
formance year, the realized LTI for the 2020-2022 performance cycle, and any buyouts vesting in 2022. The por-
tion of the Annual Incentive paid in shares for the year 2022 is disclosed using the underlying value of Novartis
shares at the date of grant, while the realized values of any other equity awards (including dividend equivalents) are
calculated using the share price on the date of vesting.
To determine the appropriateness of the 2022 CEO and executive compensation payouts under the Annual
Incentive and LTI plans, the Board of Directors and the Compensation Committee reviewed management’s perfor-
mance and contribution, taking the following into consideration:
• Operational and financial performance against targets
• Progress toward strengthening our global product portfolio
• Accomplishments across all strategic pillars, with careful attention given to ESG performance
The incentive performance outcomes, combined with base salary and other benefits, pension, and dividend equiv-
alents, resulted in 2022 total realized compensation for the CEO of CHF8 452 176.
2022 realized compensation for the CEO and other Executive Committee members
annual base
pension
Annual Incentive Long-Term
Other
salary
benefits
Incentives
compensation
LTPP –
cycle
Total realized
compensation
Equity (value
(incl. share
Currency
Cash (amount)
Amount
Cash
Equity
at vesting date)
Amount
price movement)
Executive Committee members
Vasant Narasimhan (CEO) CHF
Aggregate realized compensation of the
other Executive Committee members,
including the members who stepped down
during the financial year
CHF
Total CHF
See 2021 realized compensation for the CEO and other Executive Committee members for 2021 comparative figures.
1
Includes mandatory employer contributions of CHF 4560 for the CEO and CHF 67148 for the other current Executive Committee members paid by Novartis to governmental social
security systems. This amount is out of total employer contributions of CHF 3937537 paid in 2022 for all Executive Committee members, and provides a right to the maximum future
insured government pension benefit.
2
The portion of the Annual Incentive delivered in equity is rounded up to the nearest share, based on the closing share price on the grant date (January 25, 2023) of CHF 85.30 per
Novartis share and USD 92.81 per ADR.
3
The amounts represent the underlying share value of the 97361 LTPP PSUs vesting on January 25, 2023, to the CEO and other Executive Committee members for the 2020-2022
performance cycle and dividend equivalents for the three-year cycle (for details, see ‘’—LTPP performance outcomes’’). The taxable value is determined using the closing share price
on the day the Novartis Board of Directors approved the final LTPP performance factor (i.e., January 25, 2023) of CHF 85.30 per Novartis share and USD 92.81 per ADR. Robert
Kowalski was promoted to the Executive Committee during the course of the 2021 performance period and Victor Bulto during the course of the 2022 performance period, and as
such, the information disclosed reflects their pro-rata LTPP 2020-2022 payout attributable to the period in which they were members of the Executive Committee. Shreeram Aradhye
rejoined Novartis and Karen Hale, Aharon Gal and Fiona Marshall joined Novartis after the 2020 LTI awards were made and hence did not receive an LTPP award for the 2020-2022
performance period.
4
Includes any other perquisites, benefits in kind, and international assignment benefits as per the global mobility policy (e.g., housing, international health insurance, children’s school
fees, tax equalization). The 2022 tax payments were CHF 221633 for Richard Saynor, as well as CHF 533927 for Victor Bulto, CHF 127980 for Robert Kowalski, CHF 109966 for
Aharon Gal, and CHF 417826 for Vas Narasimhan.
5
Includes 696 vested RSUs and 2765 PSUs (for a total value of CHF 268158), which vested on March 13, 2022, to John Tsai in lieu of the LTI that he forfeited when leaving his previous
employer. Also includes 2348 vested RSUs and 1586 vested PSUs (for a total value of CHF 313815), which vested on February 13, 2022, to Richard Saynor in lieu of the LTI that he
forfeited when leaving his previous employer, and 3675 vested PSUs (CHF 287238) on January 18, 2022, to Klaus Moosmayer in lieu of the LTI he forfeited when leaving his previous
employer as well as 15 448 RSUs (CHF 1292225), which vested on December 1, 2022, to Aharon Gal in lieu of the LTI that he forfeited when leaving his previous employer.
6
All amounts are before deduction of the social security contribution and income tax due from the Executive Committee member.
7
Includes compensation of the following members who stepped down from the ECN: Richard Saynor, Sandoz CEO designate, James Bradner, former President NIBR, Susanne
Schaert, former CEO Oncology, John Tsai, former Global Head of Drug Development and Chiel Medical Ocer and Robert Weltevreden, former Head of Customer and Technology
Solutions, including the vesting of their Long-Term Incentives for 2020-2022 performance cycle, as per the plan rules. The compensation and benefits elements related to the period
after the step-down dates are reported under the ‘other 2022 compensation’ column. See “—2022 Executive Committee member departures” for details.
8
Amounts for Executive Committee members paid in USD were converted at a rate of USD 1.00 = CHF 0.9548, which is the same average exchange rate used in the Group’s 2022
consolidated financial statements (a similar rule applies to payments made in other currencies during the year).

Item 6. Directors, Senior Management and Employees
106
The table and information below provide additional details on awards granted as part of the 2020-2022 LTPP per-
formance cycle, including the number of shares awarded and delivered, following the application of the payout fac-
tor and the addition of dividend equivalent shares.
2020-2022 LTPP performance cycle
PSUs at grant
Shares delivered at vesting
Dividend
Total shares
PSUs
Performance shares
Dividend
equivalent shares
delivered at vesting
(target value
Payout factor
Performance shares
delivered at vesting
equivalent shares
delivered at vesting
(value at
PSUs
at grant date)
for ECN LTPP
delivered at vesting
(value at vesting date)
delivered at vesting
(value at vesting date)
vesting date)
(target number)
(CHF)
( of target)
(number)
(CHF)
(number)
(CHF)
(CHF)
Executive Committee members
Vasant Narasimhan
Other Executive Committee members,
including the members who stepped
down during the financial year
Total
1
Robert Kowalski and Victor Bulto joined the Executive Committee during the course of the 2020-2022 performance period. As such, the information disclosed reflects their pro-rata
LTPP 2020-2022 attributable to the period in which they were members of the Executive Committee. Karen Hale, Aharon Gal, Fiona Marshall and Shreeram Aradhye joined Novartis
after the 2020-2022 LTPP awards were made and hence did not receive an LTPP award for this performance period.
2
The shown amounts represent the underlying share value of the target number of PSUs granted to each Executive Committee member for the 2020-2022 performance period,
based on the closing share price on the grant date (January 21, 2020) of CHF 92.89 per Novartis share and USD 95.19 per ADR.
3
The shown amounts represent the underlying share value of the number of PSUs vested for the 2020-2022 performance period, based on the closing share price on the day the
Novartis Board of Directors approved the final LTPP performance payout factor (i.e., January 25, 2023) of CHF 85.30 per Novartis share and USD 92.81 per ADR.
4
Dividend equivalent shares are calculated on the dividend each member of the Executive Committee would have received, based on the actual number of shares delivered at the end
of the 2020-2022 performance period. At vesting, the dividend equivalents are credited in shares or ADRs.
5
Includes the LTPP vesting for Richard Saynor, Sandoz CEO Designate, James Bradner, former President NIBR, Susanne Schaert, former CEO Oncology, John Tsai, former Global
Head of Drug Development and Chiel Medical Ocer and Robert Weltevreden, former Head of Customer and Technology Solutions for the 2020-2022 performance cycle, as per
the plan rules.

Item 6. Directors, Senior Management and Employees
107
The table and information below provide details on the 2021 realized compensation for the CEO and other Execu-
tive Committee members, for comparative purposes.
2021 realized compensation for the CEO and other Executive Committee members
annual base
pension
Annual Incentive Long-Term Incentives
Other
salary
benefits
compensation
LTPP -
cycle
Total realized
compensation
Equity (value
(incl. share
Currency
Cash (amount)
Amount
Cash
Equity
at vesting date)
Amount
price movement)
Executive Committee members
Vasant Narasimhan (CEO) CHF
Aggregate realized compensation of the
other Executive Committee members,
including the members who stepped down
during the financial year
CHF
Total CHF
1
Includes mandatory employer contributions of CHF 5498 for the CEO and CHF 53693 for the other Executive Committee members paid by Novartis to governmental social security
systems. This amount is out of total employer contributions of CHF 4966397 paid in 2021 for all Executive Committee members, and provides a right to the maximum future insured
government pension benefit.
2
The portion of the Annual Incentive delivered in equity is rounded up to the nearest share, based on the closing share price on the grant date (January 26, 2022) of CHF 78.16 per
Novartis share and USD 84.24 per ADR.
3
The amounts represent the underlying share value of the 296 741 LTPP PSUs vested on January 22, 2022, to the CEO and other Executive Committee members for the 2019-2021
performance cycle, inclusive of earned Alcon Keep Whole awards and dividend equivalents for the three-year cycle (for details, see ‘’—LTPP performance outcomes’’). The taxable
value is determined using the closing share price on the day the Novartis Board of Directors approved the final LTPP performance factor (i.e., January 26, 2022) of CHF 78.16 per
Novartis share and USD 84.24 per ADR. Marie-France Tschudin and Robert Kowalski were promoted to the Executive Committee during the course of the 2019-2021 performance
period, and as such, the information disclosed reflects their pro-rata LTPP 2019-2021 payout attributable to the period in which they were members of the Executive Committee.
Richard Saynor and Karen Hale joined Novartis after the 2019 LTI awards were made and hence did not receive an LTPP award for the 2019-2021 performance period.
4
Includes any other perquisites, benefits in kind, and international assignment benefits as per the global mobility policy (e.g., housing, international health insurance, children’s school
fees, tax equalization). The 2021 tax payments were CHF 127009 for Mr. Saynor, as well as CHF 822808 for Susanne Schaert, and CHF 156788 for Vas Narasimhan.
5
Includes 6128 vested RSUs and 3546 PSUs (for a total value of CHF 782649), which vested partially on March 13, 2021, and partially on July 28, 2021, to John Tsai in lieu of the LTI
that he forfeited when leaving his previous employer. Also includes 2584 vested RSUs and 2043 vested PSUs (for a total value of CHF 379414), which vested on February 14, 2021,
to Mr. Saynor in lieu of the LTI that he forfeited when leaving his previous employer, and 4313 vested PSUs (CHF 370961) on January 18, 2021, to Klaus Moosmayer in lieu of the LTI
he forfeited when leaving his previous employer.
6
All amounts are before deduction of the social security contribution and income tax due from the Executive Committee member.
7
Includes the first six weeks of Karen Hale’s compensation, before her appointment to the Executive Committee, under other compensation. Comprises the compensation of Bertrand
Bodson, former Chief Data Ocer and Steven Baert, former Chief People & Organization Ocer, including the vesting of their Long-Term Incentives for 2019-2021 performance
cycle, as per the plan rules. The compensation and benefits elements related to the period after the step-down dates are reported under the other compensation column. Unvested
shares for Shannon Klinger were forfeited upon her departure from the Company. See “—2021 Executive Committee member departures” for details.
8
Amounts for Executive Committee members paid in USD were converted at a rate of USD 1.00 = CHF 0.9139, which is the same average exchange rate used in the Group’s 2021
consolidated financial statements (a similar rule applies to payments made in other currencies during the year).
Realized compensation for the CEO and other Executive Committee members for 2022 compared
with2021
The 2022 total realized compensation for the CEO was CHF 8452176. This is a reduction of 24.7% compared with
the prior year, mainly due to the lower performance payout of the 2020-2022 LTPP (57% compared with the 107%
payout for the 2019-2021 LTPP). At the end of the 2020-2022 LTPP performance cycle, the rTSR ranking for
Novartis, which is weighted 25% of the overall LTPP opportunity, was below median, which resulted in zero payout
for this measure. Payout for Net Sales CAGR performance, also weighted 25%, was significantly lower (43% com-
pared with 119% in 2019-2021), mainly driven by the impact of Covid-19 on Sales growth during 2020 and 2021.
Furthermore, the Alcon “keep-whole” awards, granted at the time of Alcon spin-o in 2019, ended with the 2019-
2021 LTPP payout.
The 2022 total realized compensation for the Executive Committee members, including the CEO, was CHF49424771.
This decrease of 12.7% compared with the prior year can be attributed to the same reasons mentioned above. For
more detail, please refer to “—LTPP performance outcomes”.

Item 6. Directors, Senior Management and Employees
108
Compensation at grant value
In accordance with the Swiss Ordinance against Excessive Compensation in Listed Companies, Novartis contin-
ues to disclose total compensation at grant value for the CEO and other Executive Committee members. The tables
below disclose the following information for the CEO and other Executive Committee members:
• Fixed 2022 compensation (base salary and benefits)
• Actual cash portion and the deferred portion granted in equity of the 2022 Annual Incentive
• 2022-2024 LTPP performance cycle awards, which are reported at target grant date value, based on the assump-
tion that the awards will vest at 100% achievement, excluding any share price movement and dividend equivalents
that may be accrued over the performance cycle. The future payout will be determined only after the performance
cycle concludes in three years (i.e., at the end of 2024), with a payout range of 0% to 200% of the target value
• Other compensation for 2022, which includes other benefits, either paid in cash or granted in equity during the year
The compensation paid, promised or granted to the members of the Executive Committee during financial year 2022
was within the amount approved by shareholders at the 2021 AGM.
To assess CEO actual pay for performance in 2022, including the Annual Incentive payout for the 2022 performance
year and the LTI payouts for the 2020-2022 performance cycle, shareholders should refer to the 2022 realized
compensation table in “—2022 realized compensation for the CEO and other Executive Committee members.”
2022 compensation at grant value for the CEO and other Executive Committee members
Fixed compensation and
Variable compensation
pension benefits
Actual compensation paid or granted for
Long-Term Incentive
- cycle
grants at target
annual base
pension
Annual Incentive
LTPP - cycle
Other
Total
salary
benefits
(performance achieved)
compensation
compensation
paid, promised
or granted
Equity
PSUs
Cash
Cash
(value at
(target value
Currency
(amount)
Amount
(amount)
grant date)
at grant date)
Amount
Amount
Executive Committee members active on December 31, 2022
Vasant Narasimhan CHF
Shreeram Aradhye (from May , )
CHF
Victor Bulto (from May , )
,
USD
Aharon Gal (from July , )
CHF
–
Karen Hale CHF
–
Harry Kirsch CHF
Robert Kowalski CHF
Steen Lang CHF
Fiona Marshall (from November , )
,
USD
–
Klaus Moosmayer CHF
Marie-France Tschudin CHF
Tot a l
Executive Committee members who stepped down during 2022
James Bradner (until October , )
,
USD
Richard Saynor (until October , )
CHF
Susanne Schaert (until April , )
CHF
John Tsai (until May , )
CHF
Robert Weltevreden (until April , )
CHF
Subtotal
Tot a l
Based on assumption of
payout at target.
Actual payout (– of
target) will be known at
the end of the three-year
cycle in January
See next page for 2021 comparative figures.
1
Includes mandatory employer contributions of CHF 4560 for the CEO and CHF 67148 for the other current Executive Committee members paid by Novartis to governmental social security systems. This amount is out of total
employer contributions of CHF 3937537 paid in 2022 for all Executive Committee members, and provides a right to the maximum future insured government pension benefit.
2
The portion of the Annual Incentive delivered in equity is rounded up to the nearest share, based on the closing share price on the grant date (January 25, 2023) of CHF 85.30 per Novartis share and USD 92.81 per ADR.
3
The amounts represent the underlying share value of the target number of PSUs granted to Executive Committee members for the 2022-2024 performance cycle, based on the closing share price on the grant date (January
26, 2022) of CHF 78.16 per Novartis share and USD 84.24 per ADR for all members.
4
Includes any other perquisites, benefits in kind, and international assignment benefits as per the global mobility policy (e.g., housing, international health insurance, children’s school fees, tax equalization). The compensation
and benefits elements related to the period after the step-down dates are also reported under ‘other 2022 compensation’.
5
All amounts are before deduction of the social security contribution and income tax due by the Executive Committee member.
6
Shreeram Aradhye received a pro-rata LTPP award of 18 905 PSUs on June 1, 2022 (at CHF 86.18 closing share price on grant date) upon joining the organization, as per contractual entitlement.
7
Victor Bulto received his 2022 LTPP grant before his appointment to Executive Committee, therefore the reported LTPP amount is pro-rated to reflect his time as Executive Committee member over the full performance cycle.
8
Amounts in USD for Victor Bulto, Fiona Marshall and James Bradner were converted at a rate of CHF 1.00 = USD 1.0473, which is the average rate used in the Group’s 2022 consolidated financial statements.
9
Aharon Gal and Fiona Marshall did not receive a pro-rata LTPP award upon joining the organization, as they received buyout grants for their forfeited awards upon joining.
10
James Bradner stepped down from the Executive Committee on October 31, 2022 and will end his notice period on October 31, 2023, in line with his contractual notice period (for more details, see “—2022 Executive
Committee member departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the performance cycle on a pro-rata basis subject to the plan rules.
11
Richard Saynor left the Executive Committee on October 25, 2022. The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the performance cycle, subject to the plan rules.
12
Susanne Schaert stepped down from the Executive Committee on April 4, 2022 and will end her notice period on April 30, 2023, in line with her contractual notice period (for more details, see “—2022 Executive Committee
member departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the performance cycle on a pro-rata basis subject to the plan rules.
13
John Tsai stepped down from the Executive Committee on May 15, 2022 and will end his notice period on May 15, 2023, in line with his contractual notice period (for more details, see “—2022 Executive Committee member
departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the performance cycle on a pro-rata basis subject to the plan rules.
14
Robert Weltevreden stepped down from the Executive Committee on April 4, 2022 and will end his notice period on April 30, 2023, in line with his contractual notice period (for more details, see “—2022 Executive Committee
member departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the performance cycle on a pro-rata basis subject to the plan rules.
Item 6. Directors, Senior Management and Employees
109
2021 compensation at grant value for the CEO and other Executive Committee members
For comparative purposes, the table below provides the compensation at grant value for 2021.
Executive Committee member compensation at grant for financial year 2021
Fixed compensation and
Variable compensation
pension benefits
Actual compensation paid or granted for
Long-Term Incentive
- cycle
grants at target
annual base
pension
Annual Incentive
LTPP
Other
Total
salary
benefits
(performance achieved)
- cycle
compensation
compensation
paid, promised
or granted
Equity
PSUs
Cash
Cash
(value at
(target value
Currency
(amount)
Amount
(amount)
grant date)
at grant date)
Amount
Amount
Executive Committee members active on December 31, 2021
Vasant Narasimhan CHF
James Bradner
USD
Karen Hale (from May , )
CHF
Harry Kirsch CHF
Robert Kowalski (from September , )
CHF
Steen Lang CHF
Klaus Moosmayer CHF
Richard Saynor CHF
Susanne Schaert CHF
John Tsai CHF
Marie-France Tschudin CHF
–
Robert Weltevreden CHF
–
Tot a l
Executive Committee members who stepped down during 2021
Steven Baert (until June , )
CHF
–
Bertrand Bodson (until January , )
CHF
–
–
Shannon Thyme Klinger (until March , )
CHF
–
–
Subtotal
Tot a l
Based on assumption of
payout at target.
Actual payout (– of
target) will be known at
the end of the three-year
cycle in January
1
Includes mandatory employer contributions of CHF 5498 for the CEO and CHF 53693 for the other Executive Committee members paid by Novartis to governmental social security systems. This amount is out of total
employer contributions of CHF 4966 397 paid in 2021 for all Executive Committee members, and provides a right to the maximum future insured government pension benefit.
2
The portion of the Annual Incentive delivered in equity is rounded up to the nearest share, based on the closing share price on the grant date (January 26, 2022) of CHF 78.16 per Novartis share and USD 84.24 per ADR.
3
The amounts represent the underlying share value of the target number of PSUs granted to Executive Committee members for the 2021-2023 performance cycle, based on the closing share price on the grant date (January
20, 2021) of CHF 86.01 per Novartis share and USD 96.92 per ADR for all members.
4
Includes any other perquisites, benefits in kind, and international assignment benefits as per the global mobility policy (e.g., housing, international health insurance, children’s school fees, tax equalization). The compensation
and benefits elements related to the period after the step-down dates are also reported under ‘other 2021 compensation’.
5
All amounts are before deduction of the social security contribution and income tax due by the Executive Committee member.
6
Amounts in USD for James Bradner were converted at a rate of CHF 1.00 = USD 1.0942, which is the average rate used in the Group’s 2021 consolidated financial statements.
7
Karen Hale received a pro-rata LTPP award of 18639 PSUs on Apr-2, 2021 (at CHF 81.15 share price at grant) upon joining the organization, as per contractual entitlement. The other compensation amount includes the first six
weeks of compensation before her appointment to the Executive Committee.
8
Robert Kowalski received his 2021 LTPP grant before his appointment to Executive Committee, therefore the reported LTPP amount is pro-rated to reflect his time as Executive Committee member over the full performance
cycle.
9
Steven Baert left the Executive Committee on June 30, 2021 and ended his notice period on September 30, 2021, in line with his reduced contractual notice period (for more details, see “—2021 Executive Committee member
departures”). He received his 2021 Annual Incentive 100% in cash on a pro-rata basis, and the LTPP grant for the 2021-2023 performance cycle, included in the table above, will vest at the end of the performance cycle on a
pro-rata basis subject to the plan rules.
10
Bertrand Bodson left the Executive Committee on January 31, 2021 and ended his notice period on November 30, 2021, in line with his reduced contractual notice period (for more details, see “—2021 Executive Committee
member departures”). He received his 2021 Annual Incentive 100% in cash on a pro-rata basis, and no LTPP was granted for the 2021-2023 performance cycle.
11
Shannon Klinger resigned as Chief Legal Ocer as of March 15, 2021, and left the Company on May 31, 2021, in line with her reduced contractual notice period (for more details, see “—2021 Executive Committee member
departures”). The 2021 Annual Incentive and LTPP 2021-2023 cycle grant (23 586 PSUs), displayed at pro-rata value for the time she was in her role in 2021, were forfeited in full upon her departure.
Compensation at grant value for the CEO and other Executive Committee members for 2022 compared
with 2021
Compensation at grant delivered in 2022 to the CEO and the other Executive Committee members, including those
who stepped down, was CHF 70819358, which was an increase of 20.7% compared with the prior year. This increase
was driven mainly by the change in composition of the Executive Committee during 2022. Compensation at grant
for the active Executive Committee members on December 31, 2022 (11 active members versus 12 in prior year) was
CHF 49852130, which is a reduction of 3.3% from December 31, 2021.

Item 6. Directors, Senior Management and Employees
110
Additional disclosures for the CEO and other Executive Committee members
This section provides additional disclosures, including information about the shareholdings of the CEO and the
other Executive Committee members.
Malus and clawback
Consistent with our “—Executive Committee compensation philosophy and principles,” in 2022 there was no legal
or factual basis on which to exercise malus or clawback for current or former Executive Committee members.
Number of equity instruments granted to the CEO and other Executive Committee members for the
financial year 2022
Variable compensation
1
Annual Incentive
LTPP
Other
(performance achieved)
- cycle
Equity
PSUs
Equity/PSUs
(number)
(target number)
(number)
Executive Committee members active on December 31, 2022
Vasant Narasimhan
Shreeram Aradhye (from May , )
Victor Bulto (from May , )
Aharon Gal (from July , )
Karen Hale
Harry Kirsch
Robert Kowalski
Steen Lang
Fiona Marshall (from November , )
Klaus Moosmayer
Marie-France Tschudin
Total
Executive Committee members who stepped down during 2022
James Bradner (until October , )
Richard Saynor (until October , )
Susanne Schaert (until April , )
John Tsai (until May , )
Robert Weltevreden (until April , )
Subtotal
Total
See next page for 2021 comparative figures.
1
The values of the awards are reported in the table “2022 compensation at grant value for the CEO and other Executive Committee members.”
2
Vested shares, restricted shares and/or RSUs granted under the Annual Incentive for the 2022 performance period.
3
Target number of PSUs granted under the LTPP as applicable for the 2022-2024 performance cycle.
4
James Bradner stepped down from the Executive Committee on October 31, 2022 and will end his notice period on October 31, 2023, in line with his contractual notice period (for
more details, see “—2022 Executive Committee member departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the
performance cycle on a pro-rata basis subject to the plan rules.
5
Richard Saynor left the Executive Committee on October 25, 2022. The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the
performance cycle, subject to the plan rules.
6
Susanne Schaert stepped down from the Executive Committee on April 4, 2022 and will end her notice period on April 30, 2023, in line with her contractual notice period (for more
details, see “—2022 Executive Committee member departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the
performance cycle on a pro-rata basis subject to the plan rules.
7
John Tsai stepped down from the Executive Committee on May 15, 2022 and will end his notice period on May 15, 2023, in line with his contractual notice period (for more details,
see “—2022 Executive Committee member departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the performance
cycle on a pro-rata basis subject to the plan rules.
8
Robert Weltevreden stepped down from the Executive Committee on April 4, 2022 and will end his notice period on April 30, 2023, in line with his contractual notice period (for more
details, see “—2022 Executive Committee member departures”). The LTPP grant for the 2022-2024 performance cycle, included in the table above, will vest at the end of the
performance cycle on a pro-rata basis subject to the plan rules.

Item 6. Directors, Senior Management and Employees
111
Number of equity instruments granted to the CEO and other Executive Committee members for the
financial year 2021 (comparative information)
Variable compensation
1
Annual Incentive
LTPP
Other
(performance achieved)
- cycle
Equity
PSUs
Equity/PSUs
(number)
(target number)
(number)
Executive Committee members active on December 31, 2021
Vasant Narasimhan
James Bradner
Karen Hale (from May , )
Harry Kirsch
Robert Kowalski (from September , )
Steen Lang
Klaus Moosmayer
Richard Saynor
Susanne Schaert
John Tsai
Marie-France Tschudin
Robert Weltevreden
Tot al
Executive Committee members who stepped down during 2021
Steven Baert (until June , )
Bertrand Bodson (until January , )
Shannon Thyme Klinger (until March , )
Subtotal
Tot al
1
The values of the awards are reported in the table “2021 compensation at grant value for the CEO and other Executive Committee members.”
2
Vested shares, restricted shares and/or RSUs granted under the Annual Incentive for the 2021 performance period.
3
Target number of PSUs granted under the LTPP as applicable for the 2021-2023 performance cycle.
4
Steven Baert left the Executive Committee on June 30, 2021 and ended his notice period on September 30, 2021, in line with his reduced contractual notice period (for more details,
see “—2021 Executive Committee member departures”). The LTPP grant for the 2021-2023 performance cycle, included in the table above, will vest at the end of the performance
cycle on a pro-rata basis subject to the plan rules.
5
Bertrand Bodson left the Executive Committee on January 31, 2021 and ended his notice period on November 30, 2021, in line with his reduced contractual notice period (for more
details, see “—2021 Executive Committee member departures”). No LTPP was granted for the 2021-2023 performance cycle.
6
Shannon Klinger resigned as Chief Legal Ocer as of March 15, 2021, and left the Company on May 31, 2021, in line with her reduced contractual notice period (for more details, see
“—2021 Executive Committee member departures”). The LTPP 2021-2023 cycle grant (23 586 PSUs), displayed at pro-rata value for the time she was in her role in 2021, was
forfeited in full upon her departure.

Item 6. Directors, Senior Management and Employees
112
Share ownership requirements for the CEO and
other Executive Committee members
Executive Committee members are required to own at
least a minimum multiple of their annual base salary in
Novartis shares or RSUs within five years of hire or pro-
motion, as set out in the table here. In addition, the CEO
and CFO are required to hold the equity vesting under
the LTPP plan (granted since 2022) for a minimum of two
years after the vesting date. In the event of a substantial
rise or drop in the share price, the Board of Directors
may, at its discretion, amend that time period accord-
ingly.
FUNCTION OWNERSHIP LEVEL
CEO x base compensation
Other Executive Committee members x base compensation
The determination of equity amounts against the share
ownership requirements is defined to include vested and
unvested Novartis shares or American Depositary
Receipts (ADRs), together with RSUs acquired under the
Company’s compensation plans. Unvested PSUs are,
however, excluded. The determination also includes
other shares and vested options of Novartis shares or
ADRs that are owned directly or indirectly by “persons
closely linked” to an Executive Committee member. The
Compensation Committee reviews compliance with the
share ownership guideline on an annual basis.
Shares, ADRs and other equity rights owned by Executive Committee members as at December 31, 2022
1
The following table shows, in alphabetical order after the CEO, the total number of shares, ADRs and other equity
rights owned by the CEO and the other Executive Committee members and “persons closely linked” to them as at
December 31, 2022. As at December 31, 2022, no members of the Executive Committee, either individually or
together with “persons closely linked” to them, owned 1% or more of the outstanding shares or ADRs of Novartis.
As at December 31, 2022, all members who have served at least five years on the Executive Committee have met
or exceeded their personal Novartis share ownership requirements.
Equity ownership level
Total as at
Vested shares
Unvested shares
as a multiple of
Unvested target PSUs
December ,
and ADRs
and other equity rights
annual base salary
(e.g., LTPP)
Vasant Narasimhan
x
Shreeram Aradhye (from May , )
x
Victor Bulto (from May , )
x
Aharon Gal (from July , )
x
Karen Hale
x
Harry Kirsch
x
Robert Kowalski
x
Steen Lang
x
Fiona Marshall (from November , )
x
Klaus Moosmayer
x
Marie-France Tschudin
x
Subtotal
Executive Committee members who stepped down during 2022
James Bradner (until October , )
x
Richard Saynor (until October , )
x
Susanne Schaert (until April , )
x
John Tsai (until May , )
x
Robert Weltevreden (until April , )
x
Subtotal
Total
1
Includes holdings of “persons closely linked” to Executive Committee members (see the ‘persons closely linked’ definition).
2
Includes unvested shares and ADRs as well as other equity rights applicable for the determination of equity amounts for the share ownership requirements, as per the definition
above.
3
The multiple is calculated based on the full-year annual base salary and the closing share price as at the end of the 2022 financial year. The share price on the final trading day of
2022 was CHF 83.59 / USD 90.72 as at December 31, 2022.
4
The target number of PSUs is disclosed pro-rata to December 31, 2022, unless the award qualified for full vesting under the relevant plan rules.

Item 6. Directors, Senior Management and Employees
113
Fixed and variable compensation
The following table summarizes the annual base salary
and variable compensation mix at grant value for the
financial year 2022 for the CEO and other Executive
Committee members.
Annual
Variable
base salary
compensation
Vasant Narasimhan
Shreeram Aradhye (from May , )
Victor Bulto (from May , )
Aharon Gal (from July , )
Karen Hale
Harry Kirsch
Robert Kowalski
Steen Lang
Fiona Marshall (from November , )
Klaus Moosmayer
Marie-France Tschudin
Total
1
Excludes pension and other benefits and is pro-rated for ECN time.
2
See the table “2022 compensation at grant value for the CEO and other Executive
Committee members” with regard to the disclosure principles of variable
compensation.
3
Excludes members, who stepped down during the year.
Other payments to Executive Committee members
During 2022, no other payments or waivers of claims
other than those set out in the tables (including the foot-
notes) contained in this Compensation Report were
made to Executive Committee members or to “persons
closely linked” to them.
Payments to former Executive Committee
members
Under the former Executive Committee members’
contracts and in line with the Company’s LTI plan rules,
payments were made to 8 former members. Of this,
CHF993 574 relates to the vesting of LTI awards. In addi-
tion, contractual amounts totaling CHF 167 106 were
made (comprising the base salary, the Annual Incentive
and other benefits), and tax equalization on variable com-
pensation granted during international assignments
amounted to a total of CHF296 627.
No other payments (or waivers of claims) were made
to former Executive Committee members or to “persons
closely linked” to them during 2022.
Persons closely linked
“Persons closely linked” are (i) their spouse, (ii) their chil-
dren (under 18 years of age), (iii) any legal entities that
they own or otherwise control, and (iv) any legal or nat-
ural person who is acting as their fiduciary.
Note 27 to the Group’s audited consolidated
financial statements
The total expense for the year for compensation awarded
to Executive Committee and Board members, using
International Financial Reporting Standards (IFRS) mea-
surement rules, is presented in Note 27 to the Group’s
audited consolidated financial statements.
Award and delivery of equity to Novartis employees
During 2022, 12.7 million unvested restricted shares (or
ADRs), RSUs and target PSUs were granted, and 10.4
million Novartis vested shares (or ADRs) were delivered
to Novartis employees under various equity-based par-
ticipation plans. Current unvested equity instruments
(restricted shares, RSUs and target PSUs) and outstand-
ing equity options held by employees represent 1.05%
of issued shares. Novartis delivers treasury shares to
employees to fulfill these obligations and aims to oset
the dilutive impact from its equity-based participation
plans.

Item 6. Directors, Senior Management and Employees
114
Interim update regarding ongoing LTI performance cycles
Below we report how performance is tracking against our stretch targets for our ongoing LTI performance cycles.
2021-2023 LTPP
After the first two years of the three-year LTPP perfor-
mance cycle, both net sales CAGR and core operating
income CAGR are tracking behind target, driven mainly
by the impact of the safety update on Beovu. Innovation
is on track. At the end of 2022, the relative TSR for
Novartis was below median among our global healthcare
peer group.
PERFORMANCE MEASURES TRACKING
Net sales CAGR () M
Core operating income CAGR () M
Innovation () T
Relative TSR () M
CAGR = compound annual growth rate
2022-2024 LTPP
After the first year of the three-year LTPP performance
cycle, net sales CAGR is on target and core operating
income CAGR is ahead of target, while innovation perfor-
mance is on target. At the end of 2022, the relative TSR
for Novartis was below the median among our global
healthcare peer group.
PERFORMANCE MEASURES TRACKING
Net sales CAGR () T
Core operating income CAGR () T
Innovation () T
Relative TSR () M
CAGR = compound annual growth rate
T On or ahead of target M Slightly behind or behind target

Item 6. Directors, Senior Management and Employees
115
2023 Executive Committee compensation
2023 Executive Committee compensation changes
The Compensation Committee believes that the compensation system supports the company’s strategy and ensures
a strong link between pay and performance.
Following a positive vote of 90.6% in favor of our 2021 Compensation Report at the 2022 AGM, the Board and
Compensation Committee decided to make evolutionary changes to the compensation system. The aim of these
changes is to simplify and increase the transparency of our performance assessment measures, in addition to strength-
ening our focus on key strategic priorities, while also considering developments in compensation best practices.
There are also changes as a result of the Swiss Corporate Law reform, which came into eect on January 1,
2023.
Assuming that the Sandoz spin-o will occur in 2023, some impact is expected on 2023 Executive Compensation.
Annual Incentive
Following a review, the Compensation Committee
decided to adapt the financial performance in the Annual
Incentive Plan eective as of performance year 2023 as
below:
• Remove “Share of Peers” from the financial perfor-
mance measures in the Annual Incentive plan. As a
result, the Annual Incentive financial performance mea-
sures are Group Net Sales (40%), Group Operating
Income (30%) and Group Free Cash Flow
1
(30%),
thereby retaining a strong link to our key priorities
• Group financial measures will be weighted at 60% for
all Executive Committee members. Financial targets
that relate to a division or business unit, where appli-
cable, will be part of the individual strategic objectives
(40% weight)
Swiss Corporate Law reform
Following the reform of Swiss corporate law, which came
into eect on January 1, 2023, the following changes will
be made to compensation design and disclosure in the
2023 Compensation Report:
• Contracts of Executive Committee members will be
adapted so that any non-compete compensation will
not exceed the average annual compensation of the
previous three financial years
• In previous years, the Swiss regulations permitted com-
panies to award compensation of up to 40% above the
shareholder approved budget for newly appointed
members of the Executive Committee, whether that be
through internal promotions or external hires. From
2023, this additional budget of 40% will only be made
available for external appointments of Executive Com-
mittee members
These changes will require adjustments to the Articles
of Incorporation of Novartis, which will be submitted to
Novartis shareholders for their approval at the 2023
AGM.
Sandoz spin-o
In August 2022, we announced our intention to separate
Sandoz, our generics and biosimilars division, into a new
publicly traded standalone company, by way of a 100%
spin-o, subject to approval of the Novartis AG Board of
Directors and shareholders. In view of the planned spin-
o, the Compensation Committee made the following
decisions.
Sandoz CEO 2023 Annual Incentive
Based on the assumption that the spin-o will be imple-
mented in the second half of 2023, the 2023 Balanced
Scorecard for Richard Saynor, CEO designate of Sandoz,
will be adapted so that his 2023 Annual Incentive will be
based exclusively on the financial and strategic perfor-
mance of Sandoz.
Equity Restoration principles
If and when the planned spin-o occurs, holders of
vested and unvested awards in the form of Novartis
shares or ADRs will receive a dividend in kind resulting
from the spin-o in the same way as other Novartis
shareholders. Holders of unvested RSUs and PSUs will
not receive the dividend in kind resulting from the spin-
o. To compensate for the expected reduction in share-
holder value after the issue of dividend in kind, Novartis
will grant equity awards (called “Keep Whole awards”)
to its employees, including the Executive Committee
members, following the spin-o. This will be undertaken
in accordance with the Sandoz equity restoration plan,
as follows:
• The Keep Whole awards will have a value similar to the
value of the dividend in kind resulting from the spin-o
that each RSU or PSU would have received had it been
a Novartis share or ADR
• The aim of the Keep Whole awards is to ensure that
Novartis employees who have been granted RSUs or
PSUs, including Executive Committee members, are
not disadvantaged by the spin-o relative to Novartis
shareholders
More details will be shared at the time of the spin-o.
1
For the purposes of the 2023 annual incentives, free cash flow is defined as net cash flows from operating activities less purchases of property, plant and
equipment.

Item 6. Directors, Senior Management and Employees
116
2023 Executive Committee member compensation increases
Each year, we collaborate with our external advisors to benchmark the compensation levels of the members of the
Executive Committee and assess the competitiveness of their total target compensation. 2023 salary increases
have been made in line with their demonstrated performance and ability in their respective roles, and commensu-
rate to changes in responsibilities, if any, as outlined in our “—Executive Committee appointments compensation
policy”.
In general, Executive Committee members (including the CEO) will receive compensation changes applicable
to associates in Switzerland or where applicable, the US. The members who will receive an additional increase
based on the principles outlined above are mentioned below.
Karen Hale, Chief Legal Ocer
Ms. Hale, who has joined the Executive Committee in May 2021, delivered many highlights in 2022, including eec-
tively managing ongoing cases with the SEC/DOJ, creating a new, focused and ecient global legal function, pre-
paring for the Sandoz spin-o eectively, and continuing to improve the governance of the company. Eective
March 1, 2023, Ms. Hale will receive a 6% increase in annual base salary increase and a 10% increase LTI target,
as a percentage of annual base salary.
Klaus Moosmayer, Chief Ethics, Risk & Compliance Ocer
Following the implementation of the new organization structure in 2022, Mr. Moosmayer took over additional man-
agement responsibilities in the areas of HSE governance, Data Privacy, and Digital & Artificial Intelligence Compli-
ance. He demonstrated strong leadership across all responsibilities of compliance, risk, ethics, and managing
emerging issues including the company’s continuing response to the global pandemic, the lockdown in China and
crisis management related to the war in Ukraine. Eective March 1, 2023, Mr. Moosmayer will receive a 12% increase
in annual base salary increase and a 10% increase LTI target, as a percentage of annual base salary, to recognize
these additional responsibilities.
Marie-France Tschudin, President, Innovative Medicines International & Chief Commercial Ocer
Following her appointment in April 2022 as the President, Innovative Medicines International & Chief Commercial
Ocer, Ms. Tschudin eectively executed the creation of our new Innovative Medicines International organization
and Chief Commercial Oce. During the year, she designed the new organization structure, implementing a large
restructuring program and creating new therapeutic areas with clear portfolio focus, while delivering strong com-
mercial performance in Region International and ensuring growth above target for many key brands. Eective March
1, 2022, Ms. Tschudin will receive a 5% increase in annual base salary, and a 10% increase in both her Annual Incen-
tive target and LTI target, as a percentage of annual base salary, to recognize her increased responsibilities as the
Chief Commercial Ocer.
Following an assessment of their compensation competitiveness and performance, recently appointed Executive
Committee members Victor Bulto, Aharon (Ronny) Gal, and Robert Kowalski will receive a 10–20% increase in their
Annual Incentive target and/or LTI target, in line with the “—Executive Committee appointments compensation pol-
icy”. Steen Lang will also receive a 10% increase in LTI target as a result of his enhanced responsibilities in Oper-
ations.

Item 6. Directors, Senior Management and Employees
117
2022 Board compensation
Philosophy and benchmarking
Aligned with market practice in Switzerland, the Board
of Directors sets compensation for its members at a level
that allows for the attraction of high-caliber individuals,
including both Swiss and international members, who
have global experience.
Given their focus on corporate strategy, supervision
and governance, Board members do not receive variable
compensation. Each year at the AGM, shareholders are
requested to approve, in a binding vote, the total com-
pensation of the Board of Directors until the following
AGM.
The Board of Directors sets the level of compensa-
tion for its Chair and the other members to be in line with
relevant benchmark companies, including other large
Switzerland-based multinational companies such as
ABB, Credit Suisse, Holcim, Nestlé, Roche and UBS. This
peer group was chosen for Board compensation due to
the comparability of Swiss legal requirements, including
broad personal and individual liabilities under Swiss law
(and criminal liability under Swiss rules regarding board
and executive committee compensation related to the
Ordinance against Excessive Compensation in Listed
Companies), and under US law (due to the Company’s
secondary listing on the New York Stock Exchange). The
Board of Directors reviews the compensation of its mem-
bers, including the Board Chair, each year based on a
proposal by the Compensation Committee and advice
from its independent advisor, including relevant bench-
marking information. To ensure independence of deci-
sion-making, the peer group used for the Board of Direc-
tors is dierent to that used for the Executive Committee.
The Board Chair’s contract and the Board of Direc-
tors compensation policy do not provide for any termi-
nation-related payments.
Board Chair
As Board Chair, Joerg Reinhardt receives total annual
compensation valued at CHF 3.8 million. The total com-
pensation is comprised equally of cash and shares, as
follows:
• Cash compensation: CHF 1.9 million per year
• Share compensation: annual value equal to CHF 1.9
million of unrestricted Novartis shares
For 2022, the Board Chair voluntarily waived the increase
in compensation to which he is contractually entitled.
Other Boardmembers
The annual fee rates for Board membership and addi-
tional functions are included in the table below. These
were approved by the Board of Directors with eect from
the 2022 AGM. Aggregate Board compensation is
aligned with other large Swiss companies.
- AGM
CHF s annual fee
Board Chair
Board membership
Vice-Chair
Lead Independent Director
Chair of the Audit and Compliance Committee
Chair of the Compensation Committee
Chair of the following committees:
• Governance, Nomination and
Corporate Responsibilities Committee
• Science & Technology Committee
• Risk Committee
Membership of the Audit
and Compliance Committee
Membership of the following committees:
• Compensation Committee
• Governance, Nomination and
Corporate Responsibilities Committee
• Science & Technology Committee
• Risk Committee
In addition, the following policies apply regarding Board
compensation:
• 50% of compensation is delivered in cash, paid on a
quarterly basis in arrears. Board members may choose
to receive more of their compensation in shares instead
of cash
• At least 50% of compensation is delivered in shares in
two installments: one six months after the AGM; and
one 12 months after the AGM
Board members bear the full cost of their employee
social security contributions, if any, and do not receive
share options or pension benefits.
2023 Board compensation
In 2022, the Compensation Committee reviewed,
together with its independent advisor, the Board of Direc-
tors’ compensation system against the Swiss Market
Index. They found that the Board Chair fees and retainer
fees of the other Board members are well positioned and
competitive among the benchmarked companies in rela-
tion to the Company’s size, operational complexity and
corporate headquarter’s location. Additional information
on our Board benchmarking practices is provided in
“—2022 Board compensation.” The compensation sys-
tem and fee levels for the Board of Directors will there-
fore remain unchanged in 2023.

Item 6. Directors, Senior Management and Employees
118
Board member total compensation earned for the financial year 2022
Governance,
Audit and Sustainability Science &
Cash
Shares
Other
Total
Board Compliance Compensation and Nomination Technology Risk Shares
(CHF)
(CHF)
(CHF)
(CHF)
membership Committee Committee Committee Committee Committee (number)
(A)
(B)
(C)
(A)+(B)+(C)
Board members active on December 31, 2022
Joerg Reinhardt
Board Chair Chair
Simon Moroney Vice-Chair
Chair •
Lead Independent
Patrice Bula Director
• Chair
Nancy C. Andrews • • •
–
Ton Buechner • • Chair
Elizabeth Doherty • Chair •
–
Bridgette Heller • • • •
–
Daniel Hochstrasser •
Frans van Houten • • •
–
–
Ana de Pro Gonzalo •
• •
Andreas von Planta • • •
Charles L. Sawyers • • •
–
William T. Winters • • •
– –
Subtotal
Board members who stepped down at the 2022 AGM
Ann Fudge
–
Enrico Vanni
Subtotal
Total
See next page for 2021 comparative figures.
1
The shown amounts represent the gross number of shares delivered to each Board member in 2022 for the respective Board member’s service period. The number of shares
reported in this column represent: (i) the second and final equity installment delivered in February 2022 for the services from the 2021 AGM to the 2022 AGM, and (ii) the first of two
equity installments delivered in August 2022 for the services from the 2022 AGM to the 2023 AGM. The second and final equity installment for the services from the 2022 AGM to the
2023 AGM will take place in February 2023.
2
Includes an amount of CHF 29250 for mandatory employer contributions for all Board members paid by Novartis to Swiss governmental social security systems. This amount is out
of total employer contributions of CHF 453083 and provides a right to the maximum future insured government pension benefit for the Board members.
3
All amounts are before deduction of the social security contribution and income tax due by the Board member.
4
No additional committee fees for chairing the Science & Technology Committee were delivered to Joerg Reinhardt.
5
Until March 4, 2022.
6
From March 4, 2022.

Item 6. Directors, Senior Management and Employees
119
Board member total compensation earned for the financial year 2021
Governance,
Audit and Sustainability Science &
Cash
Shares
Other
Total
Board Compliance Compensation and Nomination Technology Risk Shares
(CHF)
(CHF)
(CHF)
(CHF)
membership Committee Committee Committee Committee Committee (number)
(A)
(B)
(C)
(A)+(B)+(C)
Board members active on December 31, 2021
Joerg Reinhardt
Chairman Chair
Vice Chairman /
Lead Independent
Enrico Vanni Director
• • •
Nancy C. Andrews • • •
–
Ton Buechner • • Chair
Patrice Bula • •
Elizabeth Doherty • Chair •
–
Ann Fudge • • •
–
Bridgette Heller • •
•
–
Frans van Houten • •
•
–
–
Simon Moroney • Chair
•
Andreas von Planta • Chair •
Charles L. Sawyers • • •
–
William T. Winters • • •
–
–
Subtotal
Board members who stepped down at the 2021 AGM
Srikant Datar
–
Subtotal –
–
–
–
–
Total
1
The shown amounts represent the gross number of shares delivered to each Board member in 2021 for the respective Board member’s service period. The number of shares
reported in this column represent: (i) the second and final equity installment delivered in February 2021 for the services from the 2020 AGM to the 2021 AGM, and (ii) the first of two
equity installments delivered in August 2021 for the services from the 2021 AGM to the 2022 AGM. The second and final equity installment for the services from the 2021 AGM to the
2022 AGM will take place in February 2022.
2
Includes an amount of CHF 25580 for mandatory employer contributions for all Board members paid by Novartis to governmental social security systems. This amount is out of total
employer contributions of CHF 435204 and provides a right to the maximum future insured government pension benefit for the Board member.
3
All amounts are before deduction of the social security contribution and income tax due by the Board member.
4
No additional committee fees for chairing the Science & Technology Committee were delivered to Joerg Reinhardt.
5
Until March 2, 2021.
6
From March 2, 2021.
7
No additonal compensation was paid for the Lead Independent Director role.

Item 6. Directors, Senior Management and Employees
120
Additional disclosures
Share ownership requirements for Board members
The Board Chair is required to own a minimum of 30 000
Novartis shares, and other members of the Board of
Directors are required to own at least 5 000 Novartis
shares within five years after joining the Board of Direc-
tors, to ensure their interests are aligned with those of
shareholders.
Board members are prohibited from hedging or
pledging their ownership positions in Novartis shares
that are part of their guideline share ownership require-
ment and are required to hold these shares for 12 months
after retiring from the Board of Directors. As at Decem-
ber 31, 2022, all current and former members of the
Board of Directors who were required to meet the mini-
mum share ownership requirements did so.
Shares, ADRs and share options owned by Board
members
The total number of vested Novartis shares and ADRs
owned by members of the Board of Directors and “per-
sons closely linked” to them as at December 31, 2022,
is shown in the table below. As at December 31, 2022,
no members of the Board, either individually or together
with “persons closely linked” to them, owned 1% or more
of the outstanding shares (or ADRs) of Novartis. As of
the same date, no members of the Board of Directors
held any share options to purchase Novartis shares.
Number of shares
at December ,
Joerg Reinhardt
Simon Moroney
Patrice Bula
Nancy C. Andrews
Ton Buechner
Elizabeth Doherty
Bridgette Heller
Daniel Hochstrasser
Frans van Houten
Ana de Pro Gonzalo
Andreas von Planta
Charles L. Sawyers
William T. Winters
Sub-Total
Board members who stepped down at the 2022 AGM
Enrico Vanni
Ann Fudge
Sub-Total
Total
1
Includes holdings of “persons closely linked” to Board members (see definition
“Persons closely linked”).
2
Each share provides entitlement to one vote.
Other payments to Board members
During 2022, no payments (or waivers of claims) other
than those set out in the Board member compensation
table titled “—Board member total compensation earned
for the financial year 2022” (including in the table foot-
notes) were made to current members of the Board or
to “persons closely linked” to them.
Payments to former Board members
During 2022, no payments (or waivers of claims) were
made to former Board members or to “persons closely
linked” to them.
Board member compensation approved by
shareholders
The total compensation earned by Board members from
the 2021 AGM to the 2022 AGM was within the amount
approved by shareholders at the 2021 AGM.

RISK MANAGEMENT PRINCIPLES
• Rigorous performance man-
agement process, with approval
of targets and evaluation of
performance for the CEO by
the Board of Directors
• Balanced mix of short-term
and long-term variable com-
pensation elements
• Values and Behaviors are a
key component of the Annual
Incentive and are embedded in
our culture
• Clawback and malus principles
apply to all elements of the
variable compensation
• Performance-vesting Long-
Term Incentives only, with
three-year cycles
• All variable compensation is
capped at 200% of target
• Contractual notice period of
12months
• Post-contractual non-compete
period is limited to a maximum
of 12 months from the end of
employment. Resulting com-
pensation, if applicable, will
not exceed the average annual
compensation (annual base
salary plus Annual Incentive)
of the previous three financial
years
• Good and bad leaver
provisions apply to variable
compensation of leavers
• No severance payments or
change-of-control clauses
• Share ownership requirements;
no hedging or pledging of
Novartis share ownership
• No loans granted to current or
former members of the Execu-
tive Committee and the Board
of Directors or to “persons
closely linked” to them
Item 6. Directors, Senior Management and Employees
121
Compensation governance
Legal framework
The Swiss Code of Obligations and the corporate gov-
ernance guidelines of the SIX Swiss Exchange require
listed companies to disclose certain information about
the compensation of board and executive committee
members, their equity participation, and loans made to
them. This Annual Report fulfills that requirement in addi-
tion to being in line with the principles of the Swiss Code
of Best Practice for Corporate Governance of the Swiss
Business Federation (economiesuisse). For more details,
please refer to “—Corporate Governance” in Section 6C
of this Report.
Risk management principles
The Compensation Committee, with support from its
independent advisor, reviews market trends in compen-
sation, and changes in corporate governance rules and
best practices. Together with the Risk Committee, it also
reviews the Novartis compensation systems to ensure
that they do not encourage inappropriate or excessive
risk-taking, and instead encourage behaviors that sup-
port sustainable value creation. A summary of the risk
management principles is outlined below.
Compensation decision-making authorities
Authority for decisions related to compensation is gov-
erned by the Articles of Incorporation, Board Regulations
and the Compensation Committee Charter, which are all
published on the Company website: www.novartis.com/
investors/company-overview/corporate-governance.
The Compensation Committee serves as the supervi-
sory and governing body for compensation policies and
plans within Novartis, and has overall responsibility for
determining, reviewing and proposing compensation pol-
icies and plans for approval by the Board of Directors in
line with the Compensation Committee Charter. The dis-
cussions and conclusions of each committee meeting
are delivered to the full Board of Directors. A summary
of the compensation decision-making authorities is set
out below.
Compensation authorization levels within the
parameters set by the shareholders’ meeting
DECISION ON DECISIONMAKING AUTHORITY
Compensation of Board Chair and Board of Directors
other Board members
Compensation of CEO Board of Directors
Compensation of other Executive Compensation Committee
Committee members
Committee member independence
The Compensation Committee is composed exclusively
of members of the Board of Directors who meet the inde-
pendence criteria set forth in the Board Regulations. From
the 2022 AGM, the Compensation Committee consisted
of the following four members: Simon Moroney (as Chair),
Patrice Bula, Bridgette Heller, and William Winters.
Role of the Compensation Committee’s
independent advisor
The independent external compensation advisor sup-
ports the committee in determining the design and imple-
mentation of compensation and benefits.
The Compensation Committee retained Mercer
Limited, which was appointed in July 2017, as its inde-
pendent external advisor until June 2022. As part of its
normal governance practices, and with a view to ensur-
ing the independence of the advisor, the Compensation
Committee considered a change in the Committee advi-
sor. To inform this decision, it conducted a market review
of compensation advisors, with a focus on companies
with extensive experience in European and US markets.
Following a tendering process and an analysis to ensure
that there were no conflicts of interest, the Compensa-
tion Committee appointed Mitul Shah of Deloitte AG as
its independent compensation advisor with eect from
July 2022. The independent advisors from Mercer
Limited and Deloitte AG and their respective teams that
advised and supported the committee are not responsi-
ble or rewarded for work beyond support provided to the
Compensation Committee and the People & Organiza-
tion function on senior compensation.
Meetings held in 2022 and self-evaluation
In 2022, the Compensation Committee held seven for-
mal meetings. In line with prior years, it collaborated with
the Science & Technology Committee to review and
endorse, for approval by the Board of Directors, the inno-
vation targets and achievements of the Annual Incentive
and LTPP. The Compensation Committee conducted a
self-evaluation in 2022.

Item 6. Directors, Senior Management and Employees
122
Report of the statutory auditor onthe
CompensationReportof Novartis AG
To the General Meeting of Novartis AG, Basel
Opinion
We have audited the Compensation Report of Novartis AG (the
Company) for the year ended December 31, 2022. The audit
was limited to the information on compensation, loans and
advances pursuant to Art. 14-16 of the Ordinance against
Excessive Compensation in Listed Companies Limited by
Shares (Verordnung gegen übermässige Vergütungen bei
börsenkotierten Aktiengesellschaften, VegüV) namely the
tables “2022 realized compensation for the CEO and other
Executive Committee members” on pages 105-106, “2022 com-
pensation at grant value for the CEO and other Executive Com-
mittee members” on pages 108-109, “Additional disclosures for
the CEO and other Executive Committee members” on pages
110-113, as well as the “2022 Board compensation” on page 117-
118 and the “Additional disclosures” on page 120 of the Com-
pensation Report of Novartis AG for the year ended December
31, 2022, hereinafter referred to as “disclosures made on the
pages defined as subject to audit”.
In our opinion, the information on compensation, loans and
advances in the enclosed Compensation Report defined as sub-
ject to audit complies with Swiss law and Art. 14-16 VegüV.
Basis for Opinion
We conducted our audit in accordance with Swiss law and
Swiss Standards on Auditing (SACH). Our responsibilities
under those provisions and standards are further described in
the “Auditor’s Responsibilities for the Audit of the Compensa-
tion Report” section of our report. We are independent of the
Company in accordance with the provisions of Swiss law and
the requirements of the Swiss audit profession, and we have
fulfilled our other ethical responsibilities in accordance with
these requirements.
We believe that the audit evidence we have obtained is su-
cient and appropriate to provide a basis for our opinion.
Other Matter
The Compensation Report of the Company for the year ended
December 31, 2021 was audited by another auditor who
expressed an unmodified opinion on this Report on February
1, 2022.
Other Information
The Board of Directors is responsible for the other information.
The other information comprises the information included in
the annual report, but does not include the tables and disclo-
sures in the Compensation Report mentioned in the “Opinion”
paragraph of this report, the consolidated financial statements,
the financial statements and our auditor’s reports thereon.
Our opinion on the Compensation Report does not cover the
other information and we do not express any form of assurance
conclusion thereon.
In connection with our audit of the Compensation Report, our
responsibility is to read the other information and, in doing so,
consider whether the other information is materially inconsis-
tent with the audited financial information in the Compensation
Report or our knowledge obtained in the audit or otherwise
appears to be materially misstated.
If, based on the work we have performed, we conclude that
there is a material misstatement of this other information, we
are required to report that fact. We have nothing to report in
this regard.
Board of Directors’ Responsibilities for the Compensation
Report
The Board of Directors is responsible for the preparation of a
Compensation Report in accordance with the provisions of
Swiss law and the Company’s articles of incorporation, and for
such internal control as the Board of Directors determines is
necessary to enable the preparation of a Compensation Report
that is free from material misstatement, whether due to fraud
or error. The Board of Directors is also responsible for design-
ing the compensation system and defining individual compen-
sation packages.
Auditor’s Responsibilities for the Audit of the Compensation
Report
Our objectives are to obtain reasonable assurance about
whether the information on compensation, loans and advances
pursuant to Art. 14-16 VegüV is free from material misstatement,
whether due to fraud or error, and to issue an auditor’s report
that includes our opinion. Reasonable assurance is a high level
of assurance but is not a guarantee that an audit conducted in
accordance with Swiss law and SACH will always detect a
material misstatement when it exists. Misstatements can arise
from fraud or error and are considered material if, individually
or in the aggregate, they could reasonably be expected to influ-
ence the economic decisions of users taken on the basis of the
Compensation Report.
As part of an audit in accordance with Swiss law and SACH,
we exercise professional judgment and maintain professional
scepticism throughout the audit. We also:
• Identify and assess the risks of material misstatement in the
Compensation Report, whether due to fraud or error, design
and perform audit procedures responsive to those risks, and
obtain audit evidence that is sucient and appropriate to
provide a basis for our opinion. The risk of not detecting a
material misstatement resulting from fraud is higher than for
one resulting from error, as fraud may involve collusion, forg-
ery, intentional omissions, misrepresentations, or the over-
ride of internal control.
• Obtain an understanding of internal control relevant to the
audit in order to design audit procedures that are appropri-
ate in the circumstances, but not for the purpose of express-
ing an opinion on the eectiveness of the Company’s inter-
nal control.
• Evaluate the appropriateness of accounting policies used
and the reasonableness of accounting estimates and related
disclosures made.
We communicate with the Board of Directors or its relevant
committee regarding, among other matters, the planned scope
and timing of the audit and significant audit findings, including
any significant deficiencies in internal control that we identify
during our audit.
We also provide the Board of Directors or its relevant com-
mittee with a statement that we have complied with relevant
ethical requirements regarding independence, and to commu-
nicate with them all relationships and other matters that may
reasonably be thought to bear on our independence, and where
applicable, actions taken to eliminate threats or safeguards
applied.
KPMG AG
Richard Broadbelt Norman Dittes
Licensed Audit Expert Licensed Audit Expert
Auditor in charge
Basel, January 31, 2023
Governance bodies
GENERAL MEETING OF SHAREHOLDERS
Approves operating and financial review, Novartis Group consolidated financial statements, and financial
statements of Novartis AG; decides appropriation of available earnings and dividend; approves compensation
of Board and Executive Committee; elects Board members, Board Chair, Compensation Committee members,
Independent Proxy and external auditor; adopts and modifies Articles of Incorporation
EXTERNAL AUDITOR
BOARD OF DIRECTORS
AUDIT AND
COMPLIANCE
COMMITTEE
COMPENSATION
COMMITTEE
RISK
COMMITTEE
SCIENCE &
TECHNOLOGY
COMMITTEE
GOVERNANCE,
SUSTAINABILITY
AND NOMINATION
COMMITTEE
EXECUTIVE COMMITTEE
Responsible for operational management of Novartis
Sets strategic direction of Novartis, appoints and oversees key executives, approves major transactions
andinvestments, adopts and modifies Board Regulations
Provides opinion on
compliance of Novartis
Group consolidated
financial statements and
the financial statements
of NovartisAG with
applicable standards and
Swiss law, on compliance
of the Compensation
Report with applicable law,
on effectiveness of internal
controls over financial
reporting, and limited
assurance on selected
performance indicators
in the Novartis in Society
Integrated Report
Item 6. Directors, Senior Management and Employees
123
6.C Board practices
Corporate governance
Framework
Novartis is committed to eective corporate governance,
and our corporate governance framework is intended to
support sustainable financial performance and long-
term value creation for our shareholders, patients,
employees and other stakeholders based on our Values
and Behaviors.
The Novartis corporate governance principles are further
described in key governance documents, in particular
in our Articles of Incorporation and the Regulations of
the Board, the Board Committees and the Executive
Committee (“Board Regulations”) (www.novartis.com/
investors/company-overview/corporate-governance).
The Governance, Sustainability and Nomination Com-
mittee regularly reviews both the corporate governance
principles and the key governance documents against
evolving best practice standards and new developments
in line with our commitment to maintaining the highest
standards.
To better reflect its evolving role and responsibilities
in sustainability and environmental, social and gover-
nance (ESG) matters, the Board of Directors (“Board”)
amended, eective as of March 1, 2022, the Board Reg-
ulations and renamed the Governance, Nomination and
Corporate Responsibilities Committee to the Governance,
Sustainability and Nomination Committee (GSNC).

O
p
e
r
a
t
i
o
n
s
C
o
r
p
o
r
a
t
e
f
u
n
c
t
i
o
n
s
Research &
Development
S
a
n
d
o
z
I
n
n
o
v
a
t
i
v
e
M
e
d
i
c
i
n
e
s
Item 6. Directors, Senior Management and Employees
124
Group structure and shareholders
Group structure
Novartis AG and Group companies
Novartis AG, the Group’s holding company, is a corpo-
ration organized under Swiss law with issued registered
shares and registered oce at Lichtstrasse 35, CH-4056
Basel, Switzerland.
The principal subsidiaries and associated companies
of the Novartis Group are shown in “Item 18. Financial
Statements—Note 31. Principal Group subsidiaries and
associated companies.”
Divisions and business units
Novartis has two operating divisions: Innovative Medicines
(IM), which specializes in patent-protected medicines, and
Sandoz
1
, which sells generics and biosimilars. In 2022,
Novartis integrated the Pharmaceuticals and Oncology
business units under the IM Division and created two
separate commercial organizations with a stronger geo-
graphic focus – Innovative Medicines International and
Innovative Medicines US. IM is supported by the Novartis
Institutes for BioMedical Research (NIBR) and Global
Drug Development (GDD). Both operating divisions are
supported by Novartis Operations (which combines the
former Novartis Technical Operations (NTO) and Cus-
tomer & Technology Solutions (CTS) units), and corporate
functions. The latter includes the newly created Strat-
egy & Growth function, which combines corporate strat-
egy, R&D portfolio strategy and business development.
A detailed review of 2022 business results can be found
in “Item 18. Financial Statements—Note 3. Segmentation
of key figures 2022, 2021 and 2020.”
Shareholdings
Majority holdings in publicly traded Group companies
The Novartis Group owns 70.68% of Novartis India Ltd.,
with registered oce in Mumbai, India, and a listing on the
BSE (formerly known as the Bombay Stock Exchange)
(ISIN INE234A01025, symbol: HCBA). The total market
value of the 29.32% free float of Novartis India Ltd. was
USD 59.0 million on December 31, 2022, using the quoted
market share price at year-end. Applying this share price
to all the shares of the company, the market capitalization
of the whole company was USD 201.2 million, and that
of the shares owned by Novartis was USD 142.2 million.
Shareholders
Significant shareholders
According to the Share Register, as of December 31,
2022, the following registered shareholders, including
nominees and the American Depositary Share (ADS)
depositary, held more than 2% of the total share capital,
with the right to vote all their shares based on exemp-
tions granted by the Board (see “—Item 6.C Board prac-
tices—Shareholder participation—Voting rights, restric-
tions and representation—Registration restrictions”):
2
% holding of
share capital
Dec 31, 2022
Shareholders registered for their own account:
Emasan AG, Basel 3.7
UBS Fund Management (Switzerland) AG, Basel 2.3
Credit Suisse Funds AG, Zurich 2.1
% holding of
share capital
Dec 31, 2022
Shareholders registered as nominees:
Chase Nominees Ltd., London 8.4
Nortrust Nominees Ltd., London 3.8
The Bank of New York Mellon, New York 2.9
Through The Bank of New York Mellon, Everett 1.6
Through The Bank of New York Mellon, New York 0.9
Through The Bank of New York Mellon, SA/NV, Brussels 0.4
Shareholder acting as American Depositary Share (ADS) depositary:
JPMorgan Chase Bank, N.A., New York 9.4
1 On August 25, 2022, Novartis announced its intention to separate the Sandoz
business to create a standalone company by way of a 100% spin-o, with completion
expected in the second half of 2023.
2
Excluding 7.7% of the share capital held as treasury shares by Novartis AG or its fully
owned subsidiaries. As of the entry into force of the revised Swiss Code of
Obligations on January 1, 2023, Novartis ordinary shares held by certain Swiss
foundations controlled by Novartis also no longer carry the right to vote and therefore
will be treated for this calculation as treasury shares going forward.

Item 6. Directors, Senior Management and Employees
125
According to a disclosure notification filed with
NovartisAG, Norges Bank (Central Bank of Norway),
Oslo, held 2.3% of the share capital but was not regis-
tered in the Share Register as of December31, 2022.
According to a disclosure notification filed with NovartisAG
and the SIX Swiss Exchange Regulation AG, BlackRock,Inc.,
NewYork, held between 5% and 10% but was registered
with less than 2% of the share capital as of December31,2022.
Disclosure notifications pertaining to shareholdings
filed with Novartis AG and the SIX Swiss Exchange are
published on the latter’s electronic publication platform:
www.ser-ag.com/en/resources/notifications-market-par-
ticipants/significant-shareholders.html.
Duty to make an oer
According to the Swiss Federal Act on Financial Infra-
structures, anyone who – directly, indirectly or acting in
concert with third parties – acquires equity securities
exceeding 33 1/3% of the voting rights of a company
(whether or not such rights are exercisable) is required
to make an oer to acquire all listed equity securities of
that company. A company may raise this threshold up to
49% of the voting rights (“opting up”) or may, under cer-
tain circumstances, waive the threshold (“opting out”).
Novartis AG has not adopted any such measures.
Cross shareholdings
Novartis AG has no cross shareholdings in excess of
5% of capital, or voting rights with any other company.
Overview on shareholder structure
The following tables relate only to registered share-
holders and cannot be assumed to represent the entire
investor base because nominees and JPMorgan Chase
Bank, N.A., as ADS depositary, are registered as share-
holders for a large number of beneficial owners.
As of December 31, 2022, Novartis AG had approxi-
mately 186 000 registered shareholders.
Number of registered shareholders/shares
Number of
registered
% of
As of December 31, 2022
1
shareholders
share capital
1–100 34 085
0.08
101–1000 110 467
1.86
1001–10000 37 732
4.32
10001–100000 3 212
3.39
100001–1000000 475
5.85
1000001–5000000 68
5.39
5000001 or more
2
29
46.14
Total registered shareholders/shares 186 068
67.03
Unregistered shares
32.97
Total
100.00
1
At the record date of the 2022 Annual General Meeting of Shareholders (AGM),
unregistered shares amounted to 17.0%.
2
Including significant registered shareholders as listed above
Registered shareholders by type
As of December 31, 2022 Shareholders in %
Shares in %
Individual shareholders 96.71
15.61
Legal entities
1
3.25
37.69
Nominees, fiduciaries
and ADS depositary 0.04
46.70
Total 100.00
100.00
1
Excluding 7.7% of the share capital held as treasury shares by Novartis AG or its fully
owned subsidiaries. As of the entry into force of the revised Swiss Code of
Obligations on January 1, 2023, Novartis ordinary shares held by certain Swiss
foundations controlled by Novartis also no longer carry the right to vote and therefore
will be treated for this calculation as treasury shares going forward.
Registered shareholders by country
1
As of December 31, 2022 Shareholders in %
Shares in %
Belgium 0.11
0.77
France 1.97
0.36
Germany 5.72
1.82
Japan 0.17
0.45
Luxembourg 0.06
0.79
Switzerland
2
87.14
48.39
United Kingdom 0.63
23.68
United States 0.25
21.29
Other countries 3.95
2.45
Total 100.00
100.00
1
Registered shares held by nominees are shown in the country where the company/
aliate entered in the Share Register as shareholder has its registered seat.
2
Excluding 7.7% of the share capital held as treasury shares by Novartis AG or its fully
owned subsidiaries. As of the entry into force of the revised Swiss Code of
Obligations on January 1, 2023, Novartis ordinary shares held by certain Swiss
foundations controlled by Novartis also no longer carry the right to vote and therefore
will be treated for this calculation as treasury shares going forward.

Item 6. Directors, Senior Management and Employees
126
Capital structure
Share capital
As of December 31, 2022, the share capital amounted
to CHF 1 201 860 626 fully paid-in and divided into
2 403 721 252 registered shares with a nominal value of
CHF 0.50 each.
Shares are listed on the SIX Swiss Exchange (ISIN
CH0012005267, symbol: NOVN) and on the New York
Stock Exchange (NYSE) in the form of American Depositary
Receipts (ADRs) representing American Depositary
Shares (ADSs) (ISIN US66987V1098, symbol: NVS).
No authorized and conditional capital exists as of
December 31, 2022.
Shares, participation certificates,
non-voting equity securities, profit-
sharing certificates
Shares are issued as uncertificated securities (in the
sense of the Swiss Code of Obligations) and as book
entry securities (in terms of the Swiss Act on Intermedi-
ated Securities). All shares have equal voting rights and
carry equal entitlements to dividends. No participation
certificates, non-voting equity securities (Genussscheine)
or profit-sharing certificates have been issued.
Convertible securities and options
Novartis AG has not issued convertible or exchange-
able bonds, warrants, options or other securities grant-
ing rights to shares, other than options (or similar instru-
ments such as stock appreciation rights) granted under
or in connection with equity-based participation plans of
employees. Novartis AG does not grant any new stock
options under these plans.
Limitation on transferability
No transferability restrictions are imposed on shares (for
registration restrictions, see “—Item 6.C Board practices—
Shareholder participation—Voting rights, restrictions and
representation—Registration restrictions”). The registra-
tion of shareholders in the Share Register or in the ADR
register kept by JPMorgan Chase Bank, N.A., does not
aect the tradability of shares or ADRs.
Changes to share capital
Average repurchase
AGM Shareholder decision Shares canceled
share price (CHF)
1
2020 • Capital reduction by CHF 30.16 million (from CHF1 263 687 410 to CHF1 233 530 460) 60 313 900
88.18
2021 • Capital reduction by CHF 16.32 million (from CHF 1 233 530 460 to CHF 1 217 210 460) 32 640 000
80.57
• Authorization of the Board to repurchase shares up to a maximum of CHF 10 billion
between the 2021 AGM and the 2024 AGM
2022 • Capital reduction by CHF 15.35 million (from CHF 1 217 210 460 to CHF 1 201 860 626) 30 699 668
81.82
• Authorization of the Board to repurchase shares up to a maximum of CHF 10 billion
between the 2022 AGM and the 2025 AGM
2
Average repurchase
AGM Proposal to the shareholders Shares to be canceled
share price (CHF)
1
2023 • Capital reduction by CHF 63.12 million (from CHF1 201 860 626 to CHF 1 138 738 876) 126 243 500
81.56
• Authorization of the Board to repurchase shares up to a maximum of CHF 10 billion
between the 2023 AGM and the 2026 AGM
3
1
All shares were repurchased on the SIX Swiss Exchange second trading line.
2
In addition to the remaining authorization from the 2021 AGM
3
In addition to the remaining authorization from the 2022 AGM
Key Novartis share data
Issued shares
Treasury shares
Outstanding shares at December
Weighted average number of shares outstanding
1
Approximately 99 million treasury shares (2021: 102 million, 2020: 103 million) are held in Novartis entities that restrict their availability for use.

Item 6. Directors, Senior Management and Employees
127
Per-share information
1
Basic earnings per share from continuing operations (USD)
Diluted earnings per share from continuing operations (USD)
Net cash flows from operating activities from continuing operations (USD)
Year-end equity for Novartis AG shareholders (USD)
Dividend (CHF)
Dividend (USD)
1
Calculated on the weighted average number of shares outstanding, except year-end equity
2
2022: proposal to shareholders for approval at the AGM on March 7, 2023.
3
Translated into US dollars at the December 31, 2022, rate of USD 1.081 to the Swiss franc. This translation is an example only, and should not be construed as a representation that
the Swiss franc amount represents, or has been or could be converted into US dollars at that or any other rate. 2021 and 2020, dividends are translated into US dollars at the
Bloomberg Market System Rate on the payment date.
Key ratios – December 31
2022
2021
2020
Price/earnings ratio
1
28.3
8.2
26.7
Dividend yield (%)
1
3.8
3.9
3.6
1
Based on the Novartis share price at December 31 of each year
Key data on ADRs issued in the US
2022
2021
2020
Year-end ADR price (USD) 90.72
87.47
94.43
High
1
93.75
98.47
99.01
Low
1
74.61
79.70
70.67
Number of
ADRs outstanding
2
225 435 680
269 891 321
288 755 853
1
Based on daily closing prices
2
The depositary, JPMorgan Chase Bank, N.A., holds one Novartis AG share for every
ADR issued.
Share price (CHF)
2022
2021
2020
Year-end share price 83.59
80.28
83.65
High
1
87.82
86.75
95.82
Low
1
73.98
73.44
69.96
Year-end market capitalization
(USD billions)
2
191.5
196.1
214.3
Year-end market capitalization
(CHF billions)
2
177.2
179.4
188.8
1
Based on daily closing prices
2
Market capitalization is calculated based on the number of shares outstanding
(excluding treasury shares). Market capitalization in USD is based on the market
capitalization in CHF converted at the year-end CHF/USD exchange rate.

Item 6. Directors, Senior Management and Employees
128
Shareholder participation
Shareholder engagement
Shareholder engagement is fundamental to our commit-
ment to governance and transparency, and the feedback
we receive during these engagements helps us create
long-term and sustainable value.
We concentrate our outreach eorts on our largest
100 shareholders – portfolio managers, buy-side profes-
sionals, stewardship teams and ESG analysts – who rep-
resent 60% of our ownership. While the Board Chair,
CEO and CFO, together with Investor Relations, are
accountable for ensuring eective shareholder engage-
ment, other senior managers from within and outside the
Executive Committee also participate in the meetings. We
conduct regular outreach to investors throughout the
year.
TYPES OF ENGAGEMENTS SELECT EXAMPLES:
• AGM and quarterly results teleconferences (TCs)
• Bank conferences and management roadshows
• “Meet Novartis Management” capital markets event
• Governance roadshow and TCs
• Board Chair’s TCs for US and UK investors
• ESG roadshows
• Investor Update on Access & Sustainability
(formerly known as ESG Investor Day)
• Update on the new organizational model
• Update on the Sandoz business
TOPICS DISCUSSED WITH SHAREHOLDERS DURING :
GROWTH:
• Replacement power
• Growth drivers (Cosentyx, Entresto, Zolgensma, Kisqali, Kesimpta,
Leqvio)
• Policy and pricing environment
• Life cycle management
INNOVATION:
• Progress and milestones
• Data of pipeline projects
• Return on R&D investments
PRODUCTIVITY:
• Progress on financial, strategic and operational performance
• Long-term sustainability of financial performance
• Capital allocation strategy
• New organization model
• Intention to separate Sandoz business
BUILDING TRUST WITH SOCIETY AND CULTURE:
• Board accountability on ESG, and integration of ESG and
compensation
• Strong governance, enhanced process and focus on material ESG
factors, leading to improved rating agency scores
• Patient access to innovative medicines
• Learning from Novartis Access programs implemented over the
decades, including integrated sustainable business models and
access principles to help address access and inequities
• ESG targets: full carbon neutrality, patient access targets for
strategic innovative therapies, and global health flagship programs
• Progress on culture and other human capital metrics
COMPENSATION AND GOVERNANCE:
• Diversity of the Board, the Executive Committee and the Company
• Board renewal, succession planning and evaluation
• Link of compensation system to key strategic priorities
• Risk oversight
• Stakeholder expectations from the Board on ESG matters
We appreciate the value that shareholders attach to ESG
matters. We will continue to integrate ESG into our strat-
egy and to promote transparency through our compre-
hensive ESG engagement program. We have more than
doubled the number of investor engagements on ESG mat-
ters in recent years, and in 2022, our CEO led our Inves-
tor Update on Access & Sustainability (formerly known as
ESG Investor Day) for the fourth time (marking our ninth
dedicated ESG event for investors since 2014). We also
held virtual roadshows in 2022 as part of our engage
-
ment with North American, European and Asian investors.
Voting rights, restrictions and
representation
REGISTRATION
Shareholders have the right to vote and to execute all
other rights as granted under Swiss law and the Articles
of Incorporation (see, in particular, articles 17 and 18 of
the Articles of Incorporation).
Each share registered with the right to vote by the third
business day before the General Meeting entitles the holder
to one vote at General Meetings. Article 5, paragraph 2 of the
Articles of Incorporation provides that to be registered with
voting rights, a shareholder must declare that he or she acquired
the shares in his or her own name and for his or her own
account. According to article 5, paragraph 3 of the Articles of
Incorporation, the Board may register nominees with the right
to vote. The Share Register is an internal, non-public register
subject to statutory confidentiality and data privacy.
The Articles of Incorporation are available at www.
novartis.com/investors/company-overview/corpo
-
rate-governance.
REGISTRATION RESTRICTIONS
Article 5, paragraph 2 of the Articles of Incorporation provides
that no shareholder shall be registered with the right to vote
for more than 2% of the share capital. Given that shareholder
representation at General Meetings has traditionally been com-
paratively low in Switzerland, Novartis AG considers registra-
tion restrictions necessary to prevent a minority shareholder
from dominating a General Meeting. The Board may, upon
request, grant an exemption. Considerations include whether
the shareholder supports our goal of creating sustainable
value and has a long-term investment horizon. Exemptions
are in force for the registered shareholders listed in “—Item
6.C Board practices—Group structure and shareholders—
Shareholders—Significant shareholders.” Exemptions also
apply to the Novartis Foundation for Employee Participa-
tion, Basel, which as of December 31, 2022, was registered
in the Share Register with less than 2% of the share capital,
and to Norges Bank (Central Bank of Norway), Oslo, which
as of December 31, 2022, was not registered but held 2.3%
according to a disclosure notification filed with Novartis AG.
No further exemptions were requested in 2022. The same
restrictions indirectly apply to ADR holders.
Article 5, paragraph 3 of the Articles of Incorporation
provides that no nominee shall be registered with the right
to vote for more than 0.5% of the registered share capital.
The Board may, upon request, grant an exemption from this
restriction if the nominee discloses the names, addresses

Item 6. Directors, Senior Management and Employees
129
and number of shares of the persons for whose account it
holds 0.5% or more of the registered share capital. Exemp-
tions are in force for the nominees listed in “—Item 6.C Board
practices—Group structure and shareholders—Sharehold-
ers—Significant shareholders,” and for the nominee Citibank,
London, which in 2015 requested an exemption, but as of
December 31, 2022, was not registered in the Share Regis-
ter. The same restrictions indirectly apply to ADR holders.
According to article 5, paragraph 4 of the Articles of
Incorporation, shareholders, ADR holders, or nominees who
are linked to each other or who act in concert to circumvent
registration restrictions are treated as one person or nom-
inee for the purposes of the restrictions on registration.
The registration restrictions may be changed by res-
olution of the General Meeting, with approval of at least
two-thirds of the votes represented at the meeting.
The Articles of Incorporation are available at www.
novartis.com/investors/company-overview/corpo
-
rate-governance.
ATTENDANCE, REPRESENTATION AND ONLINE PLATFORM
Registered shareholders will receive personal invita-
tions to the General Meetings along with a registration/
proxy form as well as a personal one-time password and
a QR code to log in to our online platform. By returning
the registration/proxy form or using the online platform,
shareholders are able to order an admission card for the
General Meeting or appoint another shareholder or the
Independent Proxy to vote their shares on their behalf.
If the Independent Proxy is appointed, shareholders
can also give voting instructions on alternative or addi-
tional motions related to the agenda items either (i) fol-
lowing the recommendations of the Board for such alter-
native or additional motions, or (ii) opposing such
alternative or additional motions. They can also abstain
from voting.
Shareholders choosing not to receive the compre-
hensive invitation materials will be informed of upcoming
General Meetings through a letter containing the login
credentials to access the online platform as well as a ref-
erence to www.novartis.com/investors/shareholder-in-
formation/general-meetings, where all relevant informa-
tion is available.
In accordance with Swiss legislation passed in
response to the COVID-19 pandemic, and as in the pre-
vious year, physical attendance at the 2022 Annual Gen-
eral Meeting (AGM) was not possible, and shareholders
could exercise their voting rights exclusively through the
Independent Proxy.
ADR HOLDERS
ADR holders have the rights enumerated in the deposit
agreement (such as the right to give voting instruc-
tions and to receive dividends). The ADS depositary of
Novartis AG – JPMorgan Chase Bank, N.A., New York –
holds the shares underlying the ADRs and is registered
as a shareholder in the Share Register. An ADR is not a
share, and an ADR holder is not a Novartis AG shareholder.
Each ADR represents one share. ADR holders exercise
their voting rights by instructing the depositary to exer-
cise their voting rights. The ADS depositary exercises
the voting rights for registered shares underlying ADRs
for which no voting instructions have been given by pro
-
viding a discretionary proxy to an uninstructed indepen-
dent designee. Such designee has to be a shareholder.
General Meeting
CONVENING
The AGM must be held within six months after the end of
our financial year (December 31), and normally takes place
in late February/early March. Extraordinary General Meet-
ings may be requested by the Board, the external auditor, or
shareholders representing at least 10% of the share capital.
AGENDA
Shareholders representing shares with an aggregate
nominal value of at least CHF 1 million may request that
an item be included in a General Meeting agenda. Such
requests must be made in writing at least 45 days before
the meeting, specifying the requested item and proposal.
POWERS
According to article 17 of the Articles of Incorporation
(www.novartis.com/investors/company-overview/corpo-
rate-governance), the following powers are vested exclu-
sively in the General Meeting:
• Adoption and amendment of the Articles of Incorporation
• Election and removal of the Board Chair, the Board and
Compensation Committee members, the Independent
Proxy and the external auditor
• Approval of the management report and the consoli-
dated financial statements
• Approval of the financial statements of Novartis AG, and
the decision on the appropriation of available earnings
shown on the balance sheet, including dividends
• Approval of the maximum aggregate compensation of
the Board (from an AGM until the next AGM) and of the
Executive Committee (for the financial year following
the AGM). If the maximum aggregate amount of com-
pensation already approved by the AGM is not sucient
to cover the compensation of newly appointed or pro-
moted Executive Committee members, Novartis may use
up to 40% of the amount last approved for the newly
appointed or promoted Executive Committee members.
• Discharge of Board and Executive Committee members
• Decision on other matters that are reserved by law or
by the Articles of Incorporation (e.g., advisory vote on
the Compensation Report) to the General Meeting
STATUTORY QUORUMS
The General Meeting passes resolutions and elections with
the absolute majority of the votes represented at the meet-
ing. However, under article 18 of the Articles of Incorpora-
tion (www.novartis.com/investors/company-overview/
corporate-governance), the approval of two-thirds of the
votes represented at the meeting is required for:
• Alteration of the purpose of Novartis AG
• Creation of shares with increased voting powers
• Implementation of restrictions on the transfer of registe red
shares, and the removal of such restrictions
• Authorized or conditional increase of the share capital
• Increase of the share capital out of equity, by contribution
in kind, for the purpose of an acquisition of property or
the grant of special rights
• Restriction or cancellation of subscription rights
• Change of the registered oce of Novartis AG
• Dissolution of Novartis AG
In addition, the law provides for a qualified majority for
other resolutions, such as a merger or demerger.

Composition (as per December 31, 2022)
1
BOARD CHAIR: J. Reinhardt
VICECHAIR: S. Moroney
LEAD INDEPENDENT DIRECTOR: P. Bula
N. Andrews
T. Buechner
E. Doherty
B. Heller
D. Hochstrasser
1
F. van Houten
A. von Planta
2
A. de Pro Gonzalo
C. Sawyers
W. Winters
AUDIT AND
COMPLIANCE
COMMITTEE
COMPENSATION
COMMITTEE
E. Doherty (Chair)
T. Buechner
B. Heller
F. van Houten
A. de Pro Gonzalo
S. Moroney (Chair)
P. Bula
B. Heller
W. Winters
P. Bula (Chair)
B. Heller
A. von Planta
C. Sawyers
W. Winters
T. Buechner (Chair)
N. Andrews
E. Doherty
A. von Planta
A. de Pro Gonzalo
J. Reinhardt (Chair)
N. Andrews
F. van Houten
S. Moroney
C. Sawyers
GOVERNANCE,
SUSTAINABILITY
AND NOMINATION
COMMITTEE
RISK COMMITTEE SCIENCE & TECHNOLOGY
COMMITTEE
1
Eective January 1, 2023, Mr. Hochstrasser became a member of the Audit and Compliance Committee and of the Governance, Sustainability and Nomination Committee.
2
Mr. von Planta will not stand for re-election at the 2023 AGM.
Item 6. Directors, Senior Management and Employees
130
Board of Directors
Changes to the Board of Directors
Ana de Pro Gonzalo and Daniel Hochstrasser were elected
as new Board members at the 2022 AGM. Ann Fudge,
Board member since 2008, and Enrico Vanni, Board
member and Vice-Chair since 2011 and Lead Indepen-
dent Director since 2021, did not stand for re-election
at the 2022 AGM. The biographies of Ms. Fudge and Mr.
Vanni can be found in the 2021 Annual Report (pages 130
and 133), available at www.novartis.com/news/media-li-
brary/novartis-annual-report-2021.
Election and term of oce
Board members (including the Board Chair) and Com-
pensation Committee members are elected individually
by shareholders at the General Meeting for a one-year
term of oce. The term of oce expires at the end of
the next AGM.
According to article 20, paragraph 3 of the Articles
of Incorporation, a member shall not serve on the Board
for more than 12 years. Under special circumstances and
if deemed to be in the best interest of the Company, the
Board may recommend exceptions to the shareholders
(www.novartis.com/investors/company-overview/cor-
porate-governance).
The term limit supports our commitment to renew the
Board on an ongoing basis and also follows international
best practice. We believe age is still a relevant factor in
Board composition, and the GSNC will consider this and
other factors – including gender, nationality and ethnic-
ity – when evaluating candidates and exploring ways to
increase Board diversity.
Succession planning
The Board Chair, supported by the GSNC, ensures
eective succession plans for the Board, the CEO and
the Executive Committee. These plans are discussed
by the Board in private meetings. A search for a new
Board member is launched – normally with the support
of a professional executive search company – with indi-
vidual selection criteria defined based on the evolving
needs of the Company and a continuing focus on diver-
sity. The set of competencies (further explained in “—
Item 6.C Board practices—Board of Directors—Board
skills”) and a balance between continuity of experience
and fresh perspectives are also important criteria for the
GSNC when evaluating new candidates. Candidates are
interviewed by the Board Chair, members of the GSNC,
other Board members, and members of the Executive
Committee. The GSNC then makes a recommendation
to the full Board, and the Board ultimately decides who
should be proposed for election at the upcoming AGM.
The Board will propose to the shareholders a new
candidate for election at the 2023 AGM. Andreas von
Planta already announced in 2021 that he will not stand
for re-election at the 2023 AGM.
Diversity profile
p
Swiss 31%
p
American 23%
p
Dutch 11%
p
German 11%
p
British 8%
p
Spanish 8%
p
Irish 4%
p
New Zealander 4%
Gender
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
p
Male 69%
p
Female 31%
p
55–60 15%
p
61–65 62%
p
>65 23%
Tenure
p
<3y 31%
p
3–6y 31%
p
7–9y 31%
p
>9y 7%
Age
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
Nationality
1
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
1
Please note that five Board members have dual nationalities. Each of these nationalities is counted as a half in the above chart.
Board skill distribution
Medicine/healthcare/R&D 46% 6/13
Leadership/management 92% 12/13
Finance/accounting 46% 6/13
Law/regulatory/risk management 69% 9/13
Data/digital 23% 3/13
Environmental, social 38% 5/13
and governance (ESG)
Item 6. Directors, Senior Management and Employees
131
Independence
All Board members – including the Board Chair – are
non-executive and independent, pursuant to applica-
ble corporate governance rules and Novartis indepen-
dence criteria, which are outlined in Appendix II to the
Board Regulations (www.novartis.com/investors/com
-
pany-overview/corporate-governance). In particular, no
Board member is or was a member of the management
of Novartis AG or of any other Novartis Group company
in the last three financial years up to December 31, 2022,
or has or had, except for Daniel Hochstrasser, a signifi-
cant business relationship with Novartis AG or with any
other Novartis Group company. Mr. Hochstrasser fulfilled
the independence criteria following his resignation from
Bär & Karrer, a Swiss law firm that has a business rela-
tionship with Novartis, as of December 31, 2022. During
2022, Mr. Hochstrasser did not belong to any Board
committee. No separate meetings of independent Board
members were held in 2022.
The independence of Board members is assessed
annually. Each Board member completes an indepen-
dence questionnaire that is subject to review by the
GSNC. The GSNC then submits a proposal to the full
Board, and the Board determines the independence sta-
tus of each Board member.
Diversity
Diversity of gender, age, nationality, ethnicity, viewpoints,
professional backgrounds and expertise is a key factor to
success and Board eectiveness in a constantly evolving
environment. A diverse Board ensures that the appropri-
ate balance of skills, expertise, experience and cultural
background is represented to discharge its responsibil-
ities and to support long-term value creation for share-
holders, patients, employees and other stakeholders.
Diversity remains a critical area of focus for the Board,
and the GSNC is continuously looking for opportunities
to further increase the Board’s diversity when identify-
ing new Board member candidates.
Board skills
Upon proposal by the GSNC, the Board has determined
a diverse set of competencies for its members that aligns
with our status as a listed company, as well as our busi-
ness portfolio, geographic reach and culture. Based on
this set of competencies, our Board members were asked
to identify their most relevant skills highlighted by their
educational background, professional experience and
personal achievements.
The GSNC assesses the set of competencies as well
as the individual skills annually to ensure that an appro-
priate balance of skills, expertise, experience and diver-
sity is represented on the Board.
To learn more about our Board members and their
individual skills, see “—Item 6.C Board practices—Board
of Directors—Members of the Board of Directors.”

Item 6. Directors, Senior Management and Employees
132
Members of the Board of Directors
Joerg Reinhardt, Ph.D.
Chair since 2013 | Nationality: German | Year of birth: 1956
Joerg Reinhardt is a healthcare industry veteran whose career spans nearly 40 years. After receiving his
doctorate in pharmaceutical sciences, Mr. Reinhardt joined Sandoz Pharma Ltd., a predecessor to Novartis,
in 1982. He held a number of senior leadership positions at Novartis, including Chief Operating Ocer and
Head of the Vaccines and Diagnostics Division. Additionally, he led Bayer HealthCare AG as chair of the board
of management and the executive committee from 2010 to 2013.
Professional experience
• Chair of the board of management and the executive committee, Bayer HealthCare AG, Germany
(2010–2013)
• Chief Operating Ocer, Novartis AG, Switzerland (2008–2010)
• Head of the Vaccines and Diagnostics Division, Novartis AG, Switzerland (2006–2008)
• Various managerial positions at Sandoz Pharma Ltd. and Novartis AG, Switzerland (1982–2006)
Mandates
• Senate member, Helmholtz Association of German Research Centres, Germany
• Chair of the board of trustees, Institute of Molecular and Clinical Ophthalmology Basel (IOB), Switzerland
• Chair of the board of trustees, Novartis Foundation, Switzerland
• Board member, Swiss Re AG, Switzerland
• Member of the European Advisory Panel, Temasek Holdings Private Ltd., Singapore
• Board member, Lonza Group AG, Switzerland (2012–2013)
• Chair, Genomics Institute of the Novartis Research Foundation, US (2000–2010)
Education
• Doctorate in pharmaceutical sciences, Saarland University, Germany
Key skills
xMedicine/healthcare/R&D gLeadership/management lLaw/regulatory/risk management
Simon Moroney, D.Phil.
Board member since 2020 | Vice-Chair since March 4, 2022 | Nationality: German/New Zealander |
Year of birth: 1959
As co-founder and CEO of MorphoSys AG, Simon Moroney played a central role in establishing the company
as a force in the field of therapeutic antibodies, with one of the broadest pipelines of drug candidates in the
industry. Mr. Moroney holds both a doctorate and a Master of Science in chemistry.
Professional experience
• Co-founder and CEO, MorphoSys AG, Germany (1992–2019)
• Research associate, Department of Pharmacology, University of Cambridge, UK (1991–1992)
• Assistant professor, Department of Chemistry, University of British Columbia, Canada (1989–1990)
Mandates
• Chair of the board of directors and the Remuneration and Nomination Committee, Biotalys NV, Belgium
Education
• Doctorate in chemistry, University of Oxford, UK
• Master of Science in chemistry, University of Waikato, New Zealand
Key skills
gLeadership/management xMedicine/healthcare/R&D lLaw/regulatory/risk management

Item 6. Directors, Senior Management and Employees
133
Nancy C. Andrews, M.D., Ph.D.
Board member since 2015 | Nationality: American/Swiss | Year of birth: 1958
Nancy C. Andrews has extensive experience as a physician, scientist, professor and senior administrator at
leading academic institutions and hospitals. Her distinguished career spans more than 30 years, with
leadership roles at both Harvard Medical School and the Duke University School of Medicine. Dr. Andrews
currently chairs the board of the American Academy of Arts and Sciences, and is credited with conducting
research that led to advances in understanding iron biology and iron diseases.
Professional experience
• Executive vice president and chief scientific ocer, Boston Children’s Hospital, US (2021–present)
• Dean emerita, Duke University School of Medicine, and vice chancellor emerita for academic aairs,
Duke University, US (2017–2021)
• Dean, Duke University School of Medicine, and vice chancellor for academic aairs,
Duke University, US (2007–2017)
• Professor of pediatrics, pharmacology and cancer biology, Duke University, US (2007–2021)
• Dean for basic sciences and graduate studies, Harvard Medical School, US (2003–2007)
• Director, Harvard/MIT M.D.-Ph.D. Program, US (1999–2003)
• Biomedical research investigator, Howard Hughes Medical Institute, US (1993–2006)
Mandates
• Board member, Maze Therapeutics Inc., US
• Board member and chair of the Science and Technology Committee, Charles River Laboratories
International Inc., US
• Council member, National Academy of Sciences, US
• Former council member (2013–2019) and member, National Academy of Medicine, US
• Chair of the board, American Academy of Arts and Sciences, US
• Member of the Scientific Advisory Board, Dyne Therapeutics Inc., US
• Member of the executive committee of the Corporation, Massachusetts Institute of Technology, US
(2019-2022)
• Member of the Scientific Management Review Board, National Institutes of Health, US (2014–2019)
• Board member and former chair, Burroughs Wellcome Fund, US (2011–2019)
Education
• Doctor of medicine, Harvard Medical School, US
• Doctorate in biology, Massachusetts Institute of Technology, US
• Master of Science and Bachelor of Science in molecular biophysics and biochemistry, Yale University, US
Key skills
xMedicine/healthcare/R&D gLeadership/management
Ton Buechner
Board member since 2016 | Nationality: Dutch/Swiss | Year of birth: 1965
Ton Buechner is an engineer by training who started his career in the oil and gas construction industry. Before
becoming the CEO of Sulzer AG, he held several divisional leadership roles at the company and worked in
markets including Asia. Mr. Buechner most recently served as CEO and chair of the executive board of
AkzoNobel NV, where he introduced industry-leading ESG policies.
Professional experience
• CEO and chair of the executive board, AkzoNobel NV, Netherlands (2012–2017)
• CEO, Sulzer AG, Switzerland (2007–2011)
• President, Sulzer Pumps, Switzerland (2003–2006)
• President, Sulzer Turbomachinery Services, Switzerland (2000–2002)
• Various managerial positions at Sulzer AG, China and Switzerland (1994–2000)
Mandates
• Chair of the board of directors and the sustainability board, Swiss Prime Site AG, Switzerland
• Chair of the board of directors and the Strategy and Sustainability Committee, Burckhardt Compression
AG, Switzerland
• Advisor, Ammega, Switzerland
• Member of the presidential and shareholder committees, Voith GmbH & Co. KGaA, Germany
(2014–2020)
• Member of the supervisory board, Voith GmbH & Co. KGaA, Germany (2014–2018)
Education
• Master of Business Administration, IMD business school, Switzerland
• Master of Science in civil engineering, Delft University of Technology, Netherlands
Key skills
mFinance/accounting gLeadership/management lLaw/regulatory/risk management
zEnvironmental, social and governance (ESG)

Item 6. Directors, Senior Management and Employees
134
Patrice Bula
Board member since 2019 | Lead Independent Director since March 4, 2022 | Nationality: Swiss | Year of birth: 1956
Patrice Bula has 40 years of global management experience and is a leader in the consumer goods industry
across established and emerging markets. He has served in various senior roles at Nestlé SA, including as
general manager of its businesses in China, Germany and South Africa. Most recently, he successfully led
the Nestlé Group’s brand strategies, digital marketing transformation and Nespresso business.
Professional experience
• Executive vice president and head of strategic business units, marketing, sales and Nespresso, Nestlé
SA, Switzerland (2011–2021)
• Market head of the Greater China region, Nestlé SA, Switzerland (2007–2011)
• Market head of Germany, Nestlé SA, Switzerland (2003–2007)
• Head of the confectionery and biscuits strategic business unit, Nestlé SA, Switzerland (2000–2003)
• Various managerial positions at Nestlé SA, Switzerland (1980–2000)
Mandates
• Chair, Froneri Lux Topco Sarl, Luxembourg
• Board member, Schindler AG, Switzerland
• Board member and chair of the ESG Committee, New Tiger LLC, US
• Co-chair (2020–2021) and board member (2015–2021), Cereal Partners Worldwide SA, Switzerland
(Nestlé representative)
• Board member, Froneri Lux Topco Sarl, Luxembourg (Nestlé representative) (2016–2020)
• Board member, Bobst Group SA, Switzerland (2017–2019)
• Chair, Blue Bottle Coee Inc., US (Nestlé representative) (2017–2019)
• Chair, Nestlé Nespresso SA, Switzerland (Nestlé representative) (2011–2019)
• Board member, Hsu Fu Chi Food Companies, China (Nestlé representative) (2011–2019)
Education
• Program for Executive Development, IMD business school, Switzerland
• Master’s degree in economic sciences, HEC Lausanne, Switzerland
Key skills
mFinance/accounting gLeadership/management yData/digital
Elizabeth (Liz) Doherty
Board member since 2016 | Nationality: British/Irish | Year of birth: 1957 | Audit Committee Financial Expert
Elizabeth (Liz) Doherty is an expert in finance and accounting who has broad operational experience in inter-
national consumer and retail businesses. She began her career in internal audit at UnileverPLC and has held
senior finance and accounting roles there and at other companies including Tesco PLC and Reckitt Benckiser
Group PLC.
Professional experience
• CFO (interim), Cognita Schools Ltd., UK (2014–2015)
• CFO and board member, Reckitt Benckiser Group PLC, UK (2011–2013)
• CFO (interim), City Inn, UK (2010)
• CFO, Brambles Ltd., Australia (2007–2009)
• Group international finance director, Tesco PLC, UK (2001–2007)
• Various managerial positions at Unilever PLC, UK (1981–2001)
Mandates
• Board member and chair of the Audit Committee, Corbion NV, Netherlands
• Member of the supervisory board and chair of the Audit Committee, Royal Philips NV, Netherlands
• Advisor, Anity Petcare SA and GB Foods SA, Spain
• Board member, Dunelm Group PLC, UK (2013–2019)
• Board member, HM Courts & Tribunals Service, UK (2015–2019)
• Board member, Ministry of Justice, UK (2015–2019)
• Board member, Delhaize Group, Belgium (2013–2016)
• Board member, Nokia Corp., Finland (2013–2016)
Education
• Fellow, Chartered Institute of Management Accountants, UK
• Bachelor’s degree in liberal studies in science (physics), University of Manchester, UK
Key skills
gLeadership/management mFinance/accounting lLaw/regulatory/risk management

Item 6. Directors, Senior Management and Employees
135
Bridgette Heller
Board member since 2020 | Nationality: American | Year of birth: 1961
Bridgette Heller has proven experience in the standalone divisions of companies such as Johnson & Johnson,
Merck & Co. Inc. and Danone SA, and has served on the audit committees of ADT Corp. and Tech Data Corp.
During her career, she has overseen the performance of CFOs and made decisions on strategic R&D prior-
ities. Ms.Heller is an advocate for diversity, equity and inclusion, and traveled globally to reinforce Danone’s
commitment to infant and maternal health, inclusive diversity, an equitable workforce for women, and
sustainable communities. She is co-founder and CEO of the Shirley Proctor Puller Foundation, an education
and youth empowerment nonprofit, and devotes much of her time to strengthening education and sustain-
ability in an underserved community in the US.
Professional experience
• Co-founder and CEO, Shirley Proctor Puller Foundation, US (2019–present)
• EVP and president of specialized nutrition, Danone SA, Netherlands (2017–2019)
• EVP of early life nutrition, Danone SA, Netherlands (2016–2019)
• EVP and president of consumer care, Merck & Co. Inc., US (2010–2015)
• Global president of the baby global business unit, Johnson & Johnson, US (2007–2009)
• President of the US baby, kids and wound care business and of global innovation development,
Johnson & Johnson, US (2005–2007)
• Managing partner, Heller Associates: Ideas for Growth Inc., US (2004–2005)
• CEO, Chung’s Gourmet Foods, US (2003–2004)
• Various managerial positions at Kraft Foods Inc., US (1985–2003)
Mandates
• Board member, Integral Ad Science Inc., US
• Board member, Aramark, US
• Board member, Dexcom Inc., US
• Board member, Newman’s Own Inc., US
• Member of the board of trustees, Northwestern University, US
• Member of the advisory board, Kellogg School of Management at Northwestern University, US
• Board member, Shirley Proctor Puller Foundation, US
• Board member, Newman’s Own Foundation, US
• Board member, Tech Data Corp., US (2016–2020)
• Board member, ADT Corp., US (2012–2016)
• Board member, Girls Inc., US (2002–2014)
Education
• Master’s degree in marketing and management policy, Kellogg School of Management at Northwestern
University, US
• Bachelor’s degree in economics and computer studies, Northwestern University, US
Key skills
zEnvironmental, social and governance (ESG) gLeadership/management xMedicine/healthcare/R&D
mFinance/accounting
Daniel Hochstrasser
Board member since March 4, 2022 | Nationality: Swiss | Year of birth: 1960
Daniel Hochstrasser is an independent dispute resolution specialist practicing in Zurich, Switzerland. Until
the end of 2022, he has been leading Bär & Karrer’s arbitration practice for 15 years. He frequently repre-
sented parties in complex disputes arising from matters such as M&A transactions, industrial and infrastructure
projects, and license, distribution and development agreements, particularly in the pharmaceutical industry.
In addition, he led the firm as senior partner from 2011 until 2021. He has published extensively on arbitration
and litigation, and lectures at the University of Zurich and the University of St. Gallen in Switzerland.
Professional experience
• Attorney-at-law, Daniel Hochstrasser AG, Switzerland (since January 2023)
• Attorney-at-law and partner, Bär & Karrer AG, Switzerland (1993–December 2022)
• Senior partner and chair of the board of directors, Bär & Karrer AG, Switzerland (2011-2021)
• Lawyer, District Court of Aoltern, Court of Appeals/Court of Cassation of Zurich, Switzerland
(1987–1992)
• In-house lawyer, Staubli SA, France (1986–1987)
Mandates
• Member (2015–2021) and Vice President (since 2021), ICC Court of Arbitration, France
• Member of the Ethics Court, Zurich Bar Association, Switzerland (since 2004)
• Board member, Finland Arbitration Institute, Finland (since 2020)
• Chair of the board of directors, Bär & Karrer AG, Switzerland (2011-2021)
• Member of the Court, Swiss Arbitration Chambers, Switzerland (2004–2014)
Education
• Master of Laws (LL.M.), Cornell Law School, US
• Bar examination, Switzerland
• Licentiatus iuris, University of Zurich, Switzerland
Key skills
gLeadership/management lLaw/regulatory/risk management

Item 6. Directors, Senior Management and Employees
136
Frans van Houten
Board member since 2017 | Nationality: Dutch | Year of birth: 1960
Frans van Houten is passionate about purpose-driven innovation, entrepreneurship and business transfor-
mation to drive customer value and competitiveness. Under his leadership as CEO of Royal Philips, the
company transformed into a leading health technology solutions company, leveraging data and informatics
to improve healthcare provider results, and became a forerunner across ESG dimensions, having become
carbon neutral in its operations since 2020 and recycling over 90% of its waste. Mr. van Houten was an
initiator of the World Economic Forum Compact for Responsive and Responsible Leadership as well as
founder and co-chair of the Platform to Accelerate the Circular Economy.
Professional experience
• Advisor, Royal Philips NV, Netherlands (October 2022–April 2023)
• CEO and chair of the executive committee and the board of management, Royal Philips NV, Netherlands
(2011–October 2022)
• Interim management, ING Group NV, Netherlands (2009–2010)
• CEO and chair of the management board, NXP Semiconductors NV (formerly Philips Semiconductors
NV), Netherlands (2004–2009)
• Various managerial positions at Royal Philips Electronics NV, Netherlands (1986–2004)
Mandates
• Chair of the supervisory board, Erasmus Trust Foundation, Netherlands (2014–February 2023)
• Founder and co-chair of the WEF Platform to Accelerate the Circular Economy (PACE),
Netherlands (2016-December 2022)
• Member of the steering committee, European Round Table for Industry (ERT), Belgium
(2014-November 2022)
• Chair, Graduate Entrepreneur Foundation, Netherlands
• Chair, NL2025 Foundation, Netherlands
• Vice chair and member of the supervisory board, Philips Lighting, Netherlands (2016–2017)
Education
• Master of Science in economics and business management, Erasmus University Rotterdam, Netherlands
• Bachelor of Science in economics, Erasmus University Rotterdam, Netherlands
Key skills
zEnvironmental, social and governance (ESG) gLeadership/management xMedicine/healthcare/R&D
yData/digital lLaw/regulatory/risk management
Andreas von Planta, Ph.D.
Board member since 2006 | Nationality: Swiss | Year of birth: 1955
Andreas von Planta is a leading expert in corporate governance, corporate law and stock exchange regulation.
He advises boards of public companies on corporate governance matters and is a sought-after speaker and
writer on these topics. He has co-authored the Switzerland chapter of the International Comparative Legal
Guide to Corporate Governance for many years.
Professional experience
• Senior counsel, Lenz & Staehelin, Switzerland (2017–present)
• Partner, Lenz & Staehelin, Switzerland (1988–2017)
Mandates
• Board member, Helvetia Holding AG, Switzerland
• Member of the board of trustees, Novartis Foundation, Switzerland
• Board member, Helvetia Schweizerische Lebensversicherungsgesellschaft AG, Switzerland
• Board member, Helvetia Schweizerische Versicherungsgesellschaft AG, Switzerland
• Chair, HSBC Private Bank (Suisse) SA, Switzerland
• Chair, HSBC Private Banking Holdings (Suisse) SA, Switzerland
• Board member, Socotab Frana SA, Switzerland
• Chair of the regulatory board, SIX Swiss Exchange AG, Switzerland
• Chair of the Audit Committee, International Road Transport Union, Switzerland
• Board member, Société Immobilière Quai Gustave Ador 50 SA, Switzerland
• Board member, Burberry (Suisse) SA, Switzerland (2001-2022)
• Vice chair of the board of directors, A.P. Moller Finance SA, Switzerland (1997-2022)
• Board member, Raymond Weil SA, Switzerland (2007–2018)
• Board member and former chair, Clinique Générale-Beaulieu SA, Switzerland (2008–2016)
• Board member and former chair, Schweizerische National Versicherungs AG, Switzerland (1997–2015)
• Board member, Holcim AG, Switzerland (2003–2014)
Education
• Master of Laws, Columbia Law School, US
• Bar examination, Switzerland
• Doctorate in law, University of Basel, Switzerland
• Licentiatus iuris, University of Basel, Switzerland
Key skills
zEnvironmental, social and governance (ESG) lLaw/regulatory/risk management

Item 6. Directors, Senior Management and Employees
137
Ana de Pro Gonzalo
Board member since March 4, 2022 | Nationality: Spanish | Year of birth: 1967 | Audit Committee Financial Expert
Since starting her career at Arthur Andersen, Ana de Pro Gonzalo has worked across a variety of industries,
ranging from construction and real estate to engineering and telecommunications. With deep expertise in
finance, capital markets and technology, she has held executive positions at several multinational companies.
Most recently, she spent 10 years as chief financial ocer of Amadeus IT Group, a leading software provider
for the global travel and tourism industry.
Professional experience
• Chief financial ocer, Amadeus IT Group SA, Spain (2010–2020)
• Corporate general manager, Sacyr Vallehermoso SA, Spain (2002–2010)
• Deputy general manager and finance director, Metrovacesa SA, Spain (1994–2002)
• Senior auditor, Arthur Andersen SA, Spain (1990–1994)
Mandates
• Member of the supervisory board and chair of the Audit Committee, STMicroelectronics NV, Netherlands
• Board member, National Express Group PLC, UK
• Board member, Indra Sistemas SA, Spain (2020-2022)
• Board member, Merlin Properties Socimi SA, Spain (2015–2017)
Education
• General Management Program (PDG), IESE Business School, Spain
• Bachelor of Science in business studies, Complutense University of Madrid, Spain
Key skills
gLeadership/management mFinance/accounting lLaw/regulatory/risk management
Charles L. Sawyers, M.D.
Board member since 2013 | Nationality: American | Year of birth: 1959
Charles L. Sawyers is a highly accomplished expert and leader in cancer research. As a physician and
prominent scientist, he has a deep understanding of the benefits of drugs for patients and society at large,
and the importance of access to medicines. Dr. Sawyers co-developed the Novartis cancer drug Gleevec/
Glivec and has received numerous honors and awards, including the Lasker-DeBakey Clinical Medical
Research Award.
Professional experience
• Chair of the Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, US
(2006–present)
• Professor of medicine (2008–present), and professor of cell and developmental biology (2011–present),
Weill Cornell Graduate School of Medical Sciences, US
• Investigator, Howard Hughes Medical Institute, US (2002–2006 and 2008–present)
• Associate chief, Division of Hematology-Oncology, University of California, Los Angeles, US (1996–2006)
Mandates
• Member, National Academy of Medicine, US
• Member, National Academy of Sciences, US
• Investigator, Howard Hughes Medical Institute, US
• Science advisor for the following US companies: Arsenal Capital Partners; BeiGene Ltd.; Blueprint
Medicines Corp.; Foghorn Therapeutics Inc.; Housey Pharmaceutical Research Laboratories; KSQ
Therapeutics Inc.; Nextech Invest Ltd.; ORIC Pharmaceuticals Inc.; PMV Pharmaceuticals Inc.; The
Column Group
• Member, National Cancer Advisory Board, US (2012–2020)
• President, American Association for Cancer Research, US (2013–2014)
Education
• Doctor of medicine, Johns Hopkins University School of Medicine, US
• Bachelor of Arts, Princeton University, US
Key skills
xMedicine/healthcare/R&D gLeadership/management zEnvironmental, social and governance (ESG)
Anonymous survey
• Each Board member fills out an
anonymous survey.
• A report identifying key strengths and
challenges is produced for the Board
and its committees.
Qualitative review
• Based on the results, the Board Chair
and the committee chairs each lead a
qualitative review with their colleagues
and then with the entire Board.
• In addition, the Vice-Chair leads a
qualitative review of the Board Chair’s
performance, without the Chair being
present, and then provides the Board
Chair with the Board’s feedback.
Outcome
• The last self-assessment of October
2022 determined that the Board and its
committees are functioning effectively
and efficiently.
• The feedback confirmed that the Board
has an open culture, fostering a broad
range of viewpoints.
• The results also identified key areas
on which to focus, such as further
development of Novartis strategy,
oversight of a range of challenging
technology and reorganizational
projects, and the impact of the current
geopolitical situation in Europe, the US
and China, including pricing.
Item 6. Directors, Senior Management and Employees
138
William T. Winters
Board member since 2013 | Nationality: British/American | Year of birth: 1961
William T. Winters has extensive leadership experience in the financial sector. He began his career at JPMorgan
Chase & Co. in 1983 and has held management roles across several market areas and in corporate finance.
Mr. Winters founded Renshaw Bay LLP, an alternative asset management firm, and now serves as CEO of
Standard Chartered PLC, where he is leading a digital transformation of the global bank.
Professional experience
• CEO, Standard Chartered PLC, UK (2015–present)
• Chair and CEO, Renshaw Bay LLP, UK (2011–2015)
• Co-CEO of the Investment Bank, JPMorgan Chase & Co., UK (2004–2010)
• Various managerial positions at JPMorgan Chase & Co., UK and US (1983–2004)
Mandates
• Board member, Standard Chartered Bank PLC, UK
• Member of the board of overseers, International Rescue Committee, UK
• Chair of the board of trustees, The Coronet Theatre, UK
• Commissioner, Independent Commission on Banking, UK (2010–2011)
Education
• Master of Business Administration, Wharton School of the University of Pennsylvania, US
• Bachelor’s degree in international relations, Colgate University, US
Key skills
yData/digital gLeadership/management lLaw/regulatory/risk management mFinance/accounting
Corporate Secretary
Charlotte Pamer-Wieser, Ph.D.
Self-assessment
The Board and its committees conduct a self-assessment
once a year, covering topics including Board composition,
purpose, scope and responsibilities; succession planning;
Board processes and governance; interaction between the
Board and the Executive Committee; Board meetings and
pre-reading material; team eectiveness; and Board Chair
and peer evaluation. Every third year, this process is con-
ducted by an independent external consultant. This last
occurred in 2020 with the consulting firm Egon Zehnder.

Item 6. Directors, Senior Management and Employees
139
Trainings
Our Board receives regular briefings and trainings on
ethics, risks and compliance, ESG and other relevant
topics. In 2022, each Board member completed the fol-
lowing trainings:
• Health, Safety and Environment Policy
• ‘Fit to Commit’, which focused on anti-bribery, insider
trading and procurement
• An ESG educational session conducted by an external
expert on holistic value creation
• Third Party Risk Management
Our Chief Legal Ocer also provides regular updates to
our Board members on developments related to insider
trading laws and regulations and briefs the members of
the Board and the Executive Committee on an annual
basis on their respective duties. In addition, the Com-
pany oers a broad range of external trainings to its
Board members.
Role of the Board and its committees
The Board is responsible for the overall direction
and oversight of management, and holds the ultimate
decision-making authority, with the exception of deci-
sions reserved for shareholders.
The Board has delegated certain of its duties and
responsibilities to its five committees led by a Board-elected
committee chair, as set out in the Board Regulations (www.
novartis.com/investors/company-overview/corporate-gov-
ernance). In some cases, these responsibilities are of an
advisory or preparatory nature. In other cases, the com-
mittee has decision-making power that is subject to final
Board approval, or the responsibilities have been fully del-
egated to the committee. All committees have the author-
ity to retain external consultants.
Any Board member may request a Board or committee
meeting and the inclusion of an agenda item. Before
meetings, Board members receive materials to help them
prepare for the discussions and to inform decision-mak-
ing.
Attendance at Board and Board Committee Meetings in 2022
Governance,
Audit and Sustainability Science &
Compliance Compensation and Nomination Risk Technology
Name Position Board Committee Committee Committee Committee Committee
J. Reinhardt Board Chair / /
S. Moroney Vice-Chair / / /
P. Bula Lead Independent Director / / /
N. Andrews Member / / /
T. Buechner Member / / /
E. Doherty Member / / /
B. Heller Member / / / /
F. van Houten Member / / /
D. Hochstrasser
Member /
A. von Planta Member / / /
A. de Pro Gonzalo
Member / / /
C. Sawyers Member / / /
W. Winters Member / / /
1
Ms. de Pro Gonzalo and Mr. Hochstrasser were elected at the 2022 AGM.
Further details can be found on pages 140 – 145.

Item 6. Directors, Senior Management and Employees
140
Board of Directors
Primary responsibilities
• Strategy: decides on the ultimate direction of the Group’s business (including portfolio, markets, acquisitions and divestments),
considering also key ESG aspects
• Structure and organization: determines major changes in the Group’s structure and organization
• Culture: oversees the strategy and implementation of the corporate culture
• Ethics and compliance: oversees the Group’s ethics and compliance framework, including the approval of fundamental
corporate policies such as the Novartis Code of Ethics
• Risk management: oversees the Group’s risk management system, the most significant risks, and how these risks are
managed
• Finance: determines the Group’s accounting system, financial controls and financial planning;
reviews and approves the Annual Report (including the Compensation Report)
• Non-financial reporting: reviews and approves the Group’s annual reporting on non-financial matters
• People and organization: nominates or appoints, removes, and determines responsibilities of key executives,
and succession planning
Key activities in 2022
• Oversaw the Company’s strategy to become a fully focused medicines company with leading technology in key therapeutic
and geographic areas
• Reviewed the set-up and functioning of the Executive Committee in the context of the Company’s new organizational
structure
• Reviewed the geopolitical situation in Europe, with a special focus on the impact on the Russian and Ukrainian markets
• Discussed and closely monitored the Transformation for Growth project to ensure a smooth transition and the successful
implementation of its objectives
• Received an update on the US market and our priorities to accelerate growth in Innovative Medicines and become a top
player in the market
• Received an update on the German market and the Company’s strategic ambition to become the market leader in Germany
• Received updates from Global Drug Development and Operations
• Reviewed and discussed strategic considerations around mergers and acquisitions, and the Company’s larger strategic
moves to drive sustainable growth
• Conducted detailed discussions about the strategic review of Sandoz, deciding that a separation through a 100% spin-o
would oer the best value proposition to investors (subject to shareholders approval)
• Discussed the Company’s ESG strategy, plans and developments, and attended an ESG education session on holistic value
creation
• Discussed the upcoming non-financial disclosure regulations and Novartis non-financial reporting governance
• Discussed longer-term Board succession planning and required profiles, proposing a new Board member candidate to be
elected at the 2023 AGM
• Discussed the amendment of the Articles of Incorporation of Novartis AG as part of the reform of Swiss corporate law
• Discussed and reviewed the annual Board self-evaluation
Meetings
Number of meetings held 10
Number of members 13
Approximate average duration (hours) 6:30
Meeting attendance 98.5%
The Board met ten times in 2022. This includes regular meet-
ings in January, April, June, August, October and December,
and additional special meetings to deal with ad hoc matters.
Board committees typically meet the day before the meetings
of the full Board. The Board held virtual, hybrid and physical
meetings, with participants joining in person when possible.
J. Reinhardt (Board Chair) 10
S. Moroney (Vice-Chair) 10
P. Bula (Lead Independent Director) 10
N. Andrews 9
T. Buechner 10
E. Doherty 10
B. Heller 10
D. Hochstrasser
1
8
F. van Houten 10
A. von Planta 10
A. de Pro Gonzalo
1
8
C. Sawyers 10
W. Winters 9
Documents
• Articles of Incorporation of Novartis AG
• Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
1
Ms. de Pro Gonzalo and Mr. Hochstrasser were elected at the 2022 AGM and have attended all Board meetings since their election.

Item 6. Directors, Senior Management and Employees
141
Audit and Compliance Committee
Primary responsibilities
• Supervises the external auditor, and selects and nominates the external auditor for election by the shareholders (FD)**
• Oversees Internal Audit (FD)**
• Oversees accounting policies, financial controls, and compliance with accounting and internal control standards (FD)**
• Approves financial statements for the first three quarters of each calendar year and the corresponding financial results
releases (FD)**, and reviews the annual financial statements and the corresponding financial results releases (FBA)***
• Reviews the non-financial data contained in the Group’s annual reporting (FBA)***
• Oversees compliance with laws, regulations and internal policies related to its subject matter expertise (FD)**
• Reviews updates with regards to Quality Assurance and patient safety twice a year and Health Safety & Environment once
a year (FD)**
• Reviews updates from the SpeakUp Oce twice a year (FD)**
• Reviews the Group’s tax policy every two years (FD)**
• Reviews updates in closed sessions with the Chief Financial Ocer, Chief Audit Ocer, and external auditor
Key activities in 2022
• Evaluated the performance of the external auditor KPMG during 2022
• Reviewed the accounting and financial reporting, focusing on those areas involving significant risk or judgment
• Monitored the geopolitical situation and reviewed the treasury aspects and cash collection in Russia
• Reviewed and discussed the Company’s approach to non-financial reporting and assurance
• Reviewed the timelines and milestones of the intended Sandoz spin-o
• Received an update on data privacy and its mechanisms of classifications and control
• Received a presentation on foreign exchange risk management at Novartis
• Liaised with the Risk Committee to ensure adequate oversight of the Company’s key transformation projects (Enterprise
Data Governance and Management and Lean Digital Core (LDC) program)
• Received reports and updates from Internal Audit; Quality; Ethics, Risk & Compliance; the SpeakUp Oce; Health, Safety &
Environment; and Legal, and discussedprogress on identifying and remedying the root causes of issues
Meetings
Number of meetings held 7
Number of members 5
Approximate average duration (hours) 2:35
Meeting attendance 100%
E. Doherty (Chair, Audit Committee Financial Expert) 7
T. Buechner 7
B. Heller 7
F. van Houten 7
A. de Pro Gonzalo
1
(Audit Committee Financial Expert) 5
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
*
A/P = advisory or preparatory task
**
FD = fully delegated task
***
FBA = task subject to final Board approval
1
Ms. de Pro Gonzalo became a member of the Audit and Compliance Committee after the 2022 AGM and has attended all Audit and Compliance Committee meetings since that time.

Item 6. Directors, Senior Management and Employees
142
Compensation Committee
Primary responsibilities
• Designs, reviews and recommends to the Board the compensation policies and programs (FBA)***
• Advises the Board on the compensation of Board members and the CEO (A/P)*
• Decides on the compensation of Executive Committee members (FD)**
• Prepares the Compensation Report and the Say-on-Pay brochure, and submits them to the Board for approval (FBA)***
Key activities in 2022
• Made decisions relating to Executive Committee and wider employee compensation during the year
• Established compensation to be paid for the future Sandoz board and executive committee members
• Determined the critical performance measures (including financial, strategic, operational, innovation and ESG) to be
considered in the 2022 and 2023 incentive plan targets
• Reviewed the achievement of incentive plan targets for the Executive Committee members
• Reviewed shareholder and proxy advisor feedback related to Novartis compensation practices and disclosures and to
those of peer companies
• Reviewed disclosures in the Novartis Compensation Report
• Proposed appropriate peer companies for comparisons of board and executive committee compensation, and assessed
the Company’s level of compensation against the peer group
• Reviewed incentive plan rules to secure pay-for-performance alignment while preserving market competitiveness
• Appointed a new independent advisor to the Compensation Committee
• Reflected on eectiveness of the Company’s compensation programs in view of its strategy to become a fully focused
medicines company, following announcements of the introduction of a new organizational structure and the intention to
separate the Sandoz business by way of a 100% spin-o
• Reviewed the Compensation Committee charter
Meetings
Number of meetings held 7
Number of members 4
Approximate average duration (hours) 1:40
Meeting attendance 96.5%
S. Moroney (Chair) 7
P. Bula 7
B. Heller 7
W. Winters 6
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
*
A/P = advisory or preparatory task
**
FD = fully delegated task
***
FBA = task subject to final Board approval

Item 6. Directors, Senior Management and Employees
143
Governance, Sustainability and Nomination Committee
Primary responsibilities
• Oversees the Company’s strategy, governance and progress on sustainability, including access to medicine and healthcare,
global health, environmental sustainability, human capital management and other material ESG aspects (FBA)***
• Recommends corporate governance best practices to the Board (FBA)***
• Reviews the Articles of Incorporation and Board Regulations on a periodic basis (FD)**
• Reviews the composition and size of the Board and its committees as well as the skills matrix on a regular basis (FBA)***
• Identifies new Board member candidates and recommends to the Board whether existing Board members
should stand for re-election (FBA)***
• Prepares and reviews succession plans for the Board Chair, the Vice-Chair, the Lead Independent Director,
Board members, committee members and chairs, and the CEO (FBA)***
• Reviews the independence of each Board member on an annual basis (FBA)***
• Reviews directorships and agreements of Board members for conflicts of interest, and deals with conflicts of interest (FBA)***
Key activities in 2022
• Evaluated progress on sustainability at Novartis, focusing on material ESG factors, together with targets and metrics
• Received updates on ESG and Global Health covering the Company’s ESG priorities and 5-year roadmap
• Received an update on environmental sustainability covering governance, strategy and progress against near- and longer-
term targets for carbon emissions, waste reduction and water consumption
• Received an update on human capital management covering the Company’s People & Organization strategy, key people
metrics and progress in its culture journey
• Evaluated the results of the 2022 AGM as well as investor and analyst feedback from ESG / Governance roadshows held in
2022
• Discussed and recommended to the Board amendments to the Articles of Incorporation of Novartis AG in connection with
the reform of Swiss corporate law
• Discussed candidates for the Sandoz board chair elect and the nomination process for the entire Sandoz board
• Discussed the composition of, and the succession for, the (Novartis) Board and its committees on a regular basis
Meetings
Number of meetings held 3
Number of members 5
Approximate average duration (hours) 2:00
Meeting attendance 100%
P. Bula (Chair) 3
B. Heller 3
A. von Planta 3
C. Sawyers 3
W. Winters 3
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
*
A/P = advisory or preparatory task
**
FD = fully delegated task
***
FBA = task subject to final Board approval

Item 6. Directors, Senior Management and Employees
144
Risk Committee
Primary responsibilities
• Oversees the risk management system and processes (FBA)***
• Reviews, together with management, the prioritization and handling of risks, the risk portfolio,
and actions implemented by management (FBA)***
• Performs deep dives into key risk areas and fosters a culture of smart risk-taking (FBA)***
• Reviews updates on cyber security on an annual basis (FD)**
Key activities in 2022
• Received updates on Enterprise Risk Management mitigation measures and results
• Evaluated the emerging risks associated with the current geopolitical crisis in Russia and Ukraine, and mitigation actions
• Received a presentation on launch excellence in Japan, evaluating opportunities and risks for Innovative Medicines
• Reviewed and discussed the current opportunities and risks at Global Drug Development
• Discussed the performance, risk management and transformation of Novartis Technical Operations associated supply
chain
• Received updates and closely monitored the Enterprise Data Governance and Management and the risk assessment and
mitigation of the Lean Digital Core (LDC) program
• Received a presentation on falsified medicines covering the various types of falsification and indirect import
• Evaluated the enterprise risks for Innovative Medicines in the US for 2022 related to the Transforming for Growth program,
pipeline portfolio growth and diversity in clinical trials
• Reviewed the Third-Party Risk Management (TPRM) program
• Discussed the key risks associated with Intellectual Property (IP protection, IP enforcement, third-party assertion and trade
secrets)
• Analyzed the opportunities and risks around talent management in key areas and geographies
• Received a deep-dive update on cyber security, including on data loss protection, from the Chief Security Ocer
Meetings
Number of meetings held 5
Number of members 5
Approximate average duration (hours) 1:50
Meeting attendance 100%
T. Buechner (Chair) 5
N. Andrews 5
E. Doherty 5
A. von Planta 5
A. de Pro Gonzalo 5
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
*
A/P = advisory or preparatory task
**
FD = fully delegated task
***
FBA = task subject to final Board approval
Item 6. Directors, Senior Management and Employees
145
Science & Technology Committee
Primary responsibilities
• Monitors emerging scientific, data-related, technological and research trends and issues,
and brings recommendations to the Board (FBA)***
• Informs the Board on a periodic basis about critical developments for the success of the portfolio and for scientific,
technological and research activities as well as benchmarking (A/P)*
• Assists the Board with setting the Company’s strategy for science, data, technology and research (A/P)*
• Assists the Board with oversight and evaluation of the performance of the Company’s scientific, technological
and R&D activities (FBA)***
• Reviews performance and proposed targets in the area of science, technology and research (FD)**
• Reviews other matters in relation to science, data, technology and research that the committee may,
in its own discretion, deem desirable in connection with its responsibilities (A/P)*
Key activities in 2022
• Reviewed and provided guidance on the technology strategy for the Novartis Institutes of BioMedical Research (NIBR)
• Reviewed the Company’s early clinical pipeline
• Discussed the performance of Global Drug Development and its future strategy
• Provided guidance on the build-up of the Strategy & Growth function, and discussed the Company’s innovation strategy
with the Strategy & Growth leadership
Meetings
Number of meetings held 4
Number of members 5
Approximate average duration (hours) 6:00
Meeting attendance 95%
J. Reinhardt (Chair) 4
N. Andrews 4
F. van Houten 3
S. Moroney 4
C. Sawyers 4
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
*
A/P = advisory or preparatory task
**
FD = fully delegated task
***
FBA = task subject to final Board approval

Item 6. Directors, Senior Management and Employees
146
Board Chair
The Board Chair leads the Board to represent the interests
of all stakeholders and ensures an appropriate balance of
power between the Board and the Executive Committee.
In this role, the Board Chair:
• Provides leadership to the Board
• Supports and mentors the CEO
• Ensures that the Board and its committees work
eectively
• Sets the agenda, style and tone of Board discus-
sions, promoting constructive dialogue and eective
decision-making
• Ensures onboarding programs for new Board members,
and continuing education for and specialization of all
Board members
• Ensures the Board’s annual performance evaluation
• Promotes eective relationships and communication
between Board and Executive Committee members
• Ensures eective communication with the Company’s
shareholders, other stakeholders and the public
Vice-Chair and
LeadIndependentDirector
Vice-Chair
The Vice-Chair has the following responsibilities:
• Leads the Board in case and as long as the Board Chair
is incapacitated
• Leads the yearly session of the Board members to eval-
uate the performance of the Board Chair, during which
the Board Chair is not present
The Vice-Chair also provides advice and support to the
Board Chair.
Lead Independent Director
To support adequate control mechanisms, the Board
Regulations outline the role of the Lead Independent
Director. The Lead Independent Director has the follow-
ing responsibilities:
• Chairs the sessions of the independent Board members
• Leads the independent Board members in the event
of a crisis or matter requiring their separate consider-
ation or decision
The roles of the Vice-Chair and the Lead Independent
Director can be held by two Board members or by one
Board member (combined role).
The Board appointed Simon Moroney as Vice-Chair
and Patrice Bula as Lead Independent Director, both
roles eective as of March 4, 2022.
Honorary Chairmen
Alex Krauer and Daniel Vasella were appointed Honor-
ary Chairmen in recognition of their significant achieve-
ments on behalf of Novartis. In December 2021, Mr. Krauer
passed away at the age of 90.
Mr. Vasella is not provided with Board documents
and does not attend Board meetings.
Mandates outside the Novartis Group
According to article 34, paragraph 1 of the Articles of
Incorporation (www.novartis.com/investors/company-
overview/corporate-governance), the following limitations
on mandates apply:
Maximum number
of mandates
Mandates 10
Other listed companies
1
4
1
Holding a chair position of the board of directors in other listed companies counts as
two mandates.
According to article 34, paragraph 3 of the Articles of
Incorporation (www.novartis.com/investors/company-
overview/corporate-governance), the following man
-
dates are not subject to the above-mentioned limitations:
Maximum number
of mandates
Mandates in companies that are controlled by Novartis AG No limit
Mandates held at the request of Novartis AG
or companies controlled by it 5
Mandates in associations, charitable organizations,
foundations, trusts and employee welfare foundations 10
“Mandates” means those in the supreme governing body
of a legal entity that is required to be registered in the
commercial register or a comparable foreign register.
Mandates in dierent legal entities that are under joint
control are deemed to be one mandate.

Composition (as per December 31, 2022)
Shreeram Aradhye
President, Global Drug Development
& Chief Medical Officer
Vasant (Vas) Narasimhan
Chief Executive Officer
Harry Kirsch
Chief Financial Officer
Robert (Rob) Kowalski
Chief People &
Organization Officer
Victor Bulto
President, Innovative
Medicines US
Klaus Moosmayer
Chief Ethics, Risk
& Compliance Officer
Marie-France Tschudin
President, Innovative Medicines
International & Chief
Commercial Officer
Steffen Lang
President, Operations
Aharon (Ronny) Gal
Chief Strategy & Growth Officer
Fiona H. Marshall
President, Novartis Institutes
for BioMedical Research (NIBR)
Karen L. Hale
Chief Legal Officer
Item 6. Directors, Senior Management and Employees
147
Executive Committee
Changes to the Executive Committee
Susanne Schaert, President of Novartis Oncology since
2019, stepped down from her role following the Com-
pany’s decision to integrate the Pharmaceuticals and
Oncology business units and create separate US and Inter-
national commercial organizations under the Innovative
Medicines (IM) Division, eective April 4, 2022. Marie-
France Tschudin, President of Novartis Pharmaceuticals
since 2019, was appointed President, Innovative Medicines
International & Chief Commercial Ocer, eective April 4,
2022. Victor Bulto, President, Novartis Pharmaceuticals
Corporation, US, since 2019, was appointed President,
Innovative Medicines US, eective April 4, 2022. He has
been a member of the Executive Committee since May
1, 2022. Robert Weltevreden, Head of Customer & Tech-
nology Solutions (CTS) since February 1, 2021, stepped
down from his role following the Company’s decision to
combine Novartis Technical Operations (NTO) and CTS
into a new Operations unit, eective April 4, 2022. Stef-
fen Lang, Global Head of Novartis Technical Operations
since 2017, was appointed President, Operations, eec-
tive April 4, 2022. John Tsai, Head of Global Drug Devel-
opment and Chief Medical Ocer, stepped down from
his role eective May 15, 2022. Shreeram Aradhye was
appointed President, Global Drug Development & Chief
Medical Ocer, eective May 16, 2022. Aharon (Ronny)
Gal was appointed Chief Strategy & Growth Ocer, eec-
tive July 18, 2022. From April 4, 2022, until July 17, 2022,
Lutz Hegemann, President Global Health & Sustainabil-
ity, served as ad interim Chief Strategy & Growth O-
cer but was not a member of the Executive Committee.
Richard Saynor, Chief Executive Ocer, Sandoz, stepped
down from the Executive Committee eective October
25, 2022, following his appointment as CEO designate of
the Sandoz standalone company expected to be created
in the second half of 2023. James (Jay) Bradner, Presi-
dent of the Novartis Institutes for BioMedical Research
(NIBR), stepped down from his role eective October
31, 2022. Fiona H. Marshall was appointed President of
the Novartis Institutes for BioMedical Research (NIBR),
eective November 1, 2022. The biographies of the for-
mer members of the Executive Committee can be found
in the 2021 Annual Report (pages 147 – 149), available
at www.novartis.com/news/media-library/novartis-an-
nual-report-2021.
Role of the Executive Committee
The Board has appointed the Executive Committee mem-
bers and delegated the overall responsibility for and
oversight of the operational management of Novartis to
them, including:
• Recruiting, appointing and promoting senior management
• Ensuring the ecient operation of the Group and the
achievement of optimal results
• Promoting an active internal and external communi cations
policy
• Developing policies and strategic plans for Board
approval, and implementing those approved
• Submitting the following to the Board for approval: invest-
ments, divestments, transactions, contracts and litigations
with a value exceeding USD 500 million, and capital market
and other important financing transactions, as well as all
other matters of fundamental significance to the Novartis
Group
• Preparing and submitting quarterly and annual reports
to the Board and its committees
• Informing the Board of all matters of fundamental sig-
nificance to the businesses
• Dealing with any other matters delegated by the Board
There are no contracts between Novartis and third
parties whereby Novartis would delegate any business
management tasks to such third parties.
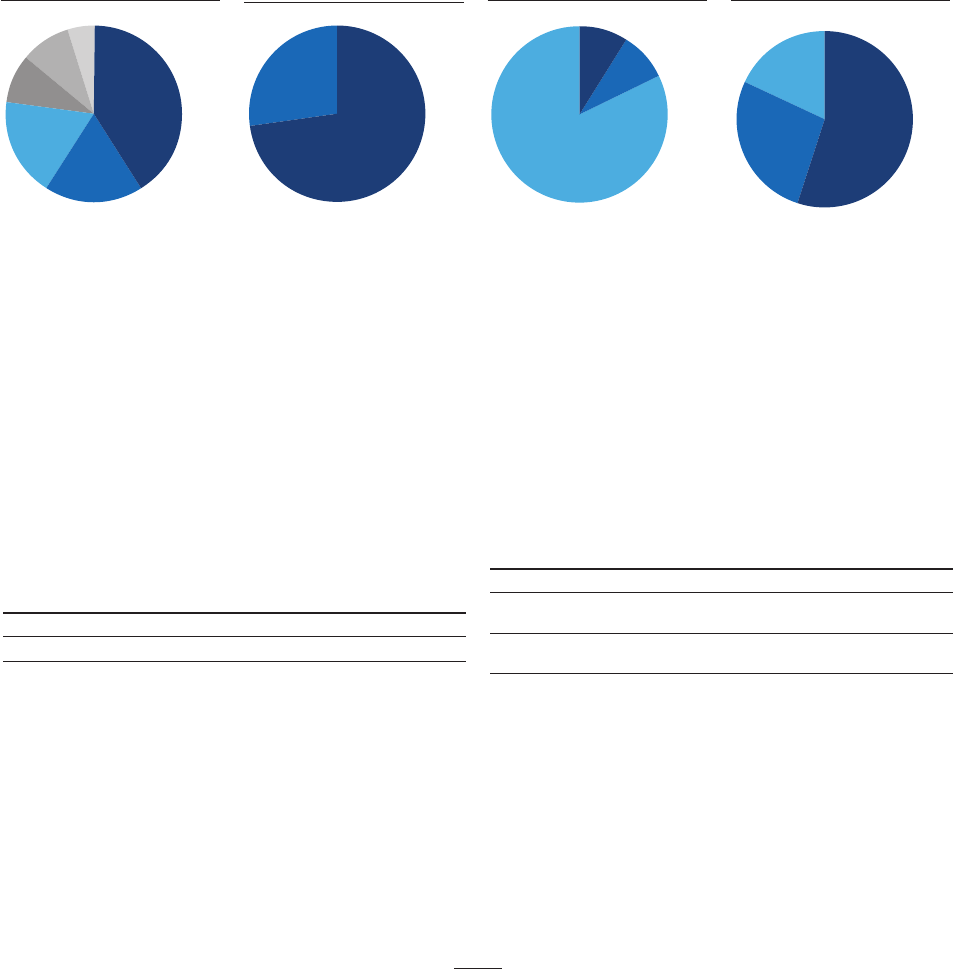
Diversity profile
p
American 41%
p
German 18%
p
Swiss 18%
p
British 9%
p
Spanish 9%
p
Israeli 5%
Gender
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
p
Male 73%
p
Female 27%
p
<45 9%
p
45–50 9%
p
>50 82%
Tenure
p
<2y 55%
p
2–4y 27%
p
>4y 18%
Age
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
Nationality
1
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
TENURE
EXECUTIVE/NON-EXECUTIVE INDEPENDENCEGENDER
AGEBACKGROUND/EXPERIENCE
NATIONALITY
1
Please note that three Executive Committee members have dual nationalities. Each of these nationalities is counted as a half in the above chart.
Item 6. Directors, Senior Management and Employees
148
CEO
With the support of the Executive Committee, the CEO is
responsible for the operational management of Novartis.
This includes eectively implementing the Company strat
-
egy, delivering financial results, and shaping a corporate
culture of empowerment and responsibility to help drive
innovation, performance and reputation.
In addition to other Board-assigned duties, the CEO
leads the Executive Committee, and is responsible for
building and maintaining an eective executive team. With
the support of the Executive Committee, the CEO is
responsible for:
• Ensuring Novartis has the capabilities to achieve its
long-term strategic objectives
• Developing robust management succession and
development plans for presentation to the Board
• Promoting eective communication with shareholders
and other stakeholders
• Ensuring Novartis conducts its business in a legal and
ethical manner
• Developing an eective risk control framework for all
business activities
• Ensuring the flow of information to the Board is accurate,
timely and clear
Diversity
The composition as of December 31, 2022, in terms of nationality, gender, age and length of tenure, is shown in
the following charts:
Mandates outside the Novartis Group
According to article 34, paragraph 2 of the Articles of
Incorporation (www.novartis.com/investors/company-
overview/corporate-governance), the following limitations
on mandates apply:
Maximum number
of mandates
Mandates 6
Other listed companies
1
2
1
Holding a chair position of the board of directors in other listed companies is not
allowed.
According to article 34, paragraph 3 of the Articles of
Incorporation (www.novartis.com/investors/company-
overview/corporate-governance), the following man-
dates are not subject to the above-mentioned limitations:
Maximum number
of mandates
Mandates in companies that are controlled by Novartis AG No limit
Mandates held at the request of Novartis AG
or companies controlled by it 5
Mandates in associations, charitable organizations,
foundations, trusts and employee welfare foundations 10
“Mandates” means those in the supreme governing body
of a legal entity that is required to be registered in the
commercial register or a comparable foreign register.
Mandates in dierent legal entities that are under joint
control are deemed to be one mandate.

Item 6. Directors, Senior Management and Employees
149
Members of the Executive Committee
Vasant (Vas) Narasimhan, M.D.
Chief Executive Ocer of Novartis since 2018 | Nationality: American | Year of birth: 1976
Professional experience
• Global Head of Drug Development and Chief Medical Ocer, Novartis AG, Switzerland (2016–2018)
• Global Head of Development, Novartis Pharmaceuticals, Switzerland (2014–2016)
• Global Head of Biopharmaceuticals and Oncology Injectables, Sandoz International, Germany (2014)
• Global Head of Development, Novartis Vaccines, US (2012–2014)
• North America Region Head, Novartis Vaccines, and US Country President, Novartis Vaccines and
Diagnostics, US (2008–2012)
• Joined Novartis in 2005
Mandates
• Member, National Academy of Medicine, US
• Chair (since December 2022) and board member (2020–2022), African Parks Network, South Africa
• Committee member, Biopharmaceutical CEOs Roundtable (BCR), International Federation of
Pharmaceutical Manufacturers & Associations (IFPMA), Switzerland
• Member of the board of fellows, Harvard Medical School, US
• Board member and treasurer, Pharmaceutical Research and Manufacturers of America (PhRMA), US
Education
• Doctor of medicine, Harvard Medical School, US
• Master’s degree in public policy, John F. Kennedy School of Government, Harvard University, US
• Bachelor’s degree in biological sciences, University of Chicago, US
Shreeram Aradhye, M.D.
President, Global Drug Development & Chief Medical Ocer since May 16, 2022 | Nationality: American |
Year of birth: 1962
Professional experience
• Executive Vice President & Chief Medical Ocer, Dicerna Pharmaceuticals, US (2020–March 2022)
• Executive Vice President & Chief Development Ocer, Axcella Health, US (2019–2020)
• Global Head, Medical Aairs and Chief Medical Ocer, Pharmaceuticals, Novartis, US & Switzerland
(2017–2019)
• Global Head, Development Franchise, Neuroscience, and US Head, Development, Novartis,
US&Switzerland (2013–2017)
• Executive Global Program Head, Multiple Sclerosis, Novartis, Switzerland (2012–2013)
• Head, Global Development India, Novartis, India (2011–2012)
• Head, Global Clinical Development & Medical Aairs, Biosimilars, Sandoz, Germany (2009–2011)
• Joined Novartis in 1999 holding positions of increasing responsibility
Education
• Chief Resident and Teaching Fellow in Internal Medicine, Newton Wellesley Hospital, US
• Resident in Internal Medicine, Newton Wellesley Hospital, US
• Fellow in Nephrology, St Luke’s Roosevelt Medical Center, US
• Resident in Internal Medicine (M.D.), All India Institute of Medical Sciences, India
• Bachelor of Medicine and Bachelor of Surgery, All India Institute of Medical Sciences, India
Victor Bulto
President, Innovative Medicines US since April 4, 2022 | Member of the Executive Committee as of May 1, 2022 |
Nationality: Spanish | Year of birth: 1978
Professional experience
• President, Novartis Pharmaceuticals Corporation, US (2019–April 2022)
• Vice President & Head US Immunology & Dermatology Franchise, US (2017–2019)
• Vice President & Head US Alcon Pharmaceuticals, US (2016–2017)
• Head Neuroscience Franchise, Region Europe, Novartis, Switzerland (2013–2016)
• Business Franchise Head Neuroscience, Novartis, Spain (2012–2013)
• Business Franchise Head Neuroscience/MS, Respiratory, Osteoarticular, Spain, Novartis (2010–2012)
• Marketing Head Respiratory, Osteoarticular, Novartis, Spain (2009–2010)
Mandates
• Board member, Biotechnology Innovation Organization (BIO), US
Education
• Master of business administration, ESADE Business School, Spain
• Master’s degree in health economics and pharmacoeconomics, Pompeu Fabra University Spain
• Master’s degree in chemical engineering, Ramon Llull University, Spain
• Bachelor’s of science degree in chemistry, Ramon Llull University, Spain

Item 6. Directors, Senior Management and Employees
150
Aharon (Ronny) Gal, Ph.D.
Chief Strategy & Growth Ocer since July 18, 2022 | Nationality: Israeli/American | Year of birth: 1966
Professional experience
• Senior analyst, US biopharmaceutical, Sanford Bernstein, US (2020–June 2022)
• Senior analyst, US specialty pharmaceuticals and Biotech, Sanford Bernstein, US (2016–2020)
• Senior analyst, US specialty pharmaceuticals and EU mid-cap pharmaceuticals, Sanford Bernstein,
US, UK (2013–2016)
• Senior analyst, US specialty pharmaceuticals, Sanford Bernstein, US (2004–2013)
• Vice president, Canon US Life Sciences, US (2003–2004)
• Consultant, team leader, manager, The Boston Consulting Group, Inc., US, Singapore, China
(1996–2002)
Mandates
• Scientific advisor, Pure Honey Technologies, US
Education
• Ph.D. in Biochemistry, Massachusetts Institute of Technology, US
• B.Sc. in Chemistry, Emory University, US
Karen L. Hale
Chief Legal Ocer of Novartis since May 15, 2021 | Nationality: American | Year of birth: 1968
Professional experience
• Vice president, deputy general counsel, AbbVie Inc., US (2019–2021)
• Vice president, chief ethics and compliance ocer, AbbVie Inc., US (2013–2019)
• Vice president, litigation and legal specialty operations, AbbVie Inc., US (2013)
• Divisional vice president, commercial litigation, Abbott Laboratories, US (2006–2012)
• Began practicing law in 1994 and joined Abbott in 1997
Education
• Bar memberships: Illinois and Virginia, US
• Juris doctor, William & Mary Law School, US
• Bachelor’s degree in economics, Duke University, US
Harry Kirsch
Chief Financial Ocer of Novartis since 2013 | Nationality: German/Swiss | Year of birth: 1965
Professional experience
• Chief Financial Ocer of the Pharmaceuticals Division (now known as the Innovative Medicines Division),
Novartis Pharmaceuticals, Switzerland (2010–2013)
• Chief Financial Ocer of Pharma Europe, Novartis Pharmaceuticals, Switzerland (2008–2010)
• Head of Business Planning & Analysis for the Pharmaceuticals Division, Novartis Pharmaceuticals,
Switzerland (2005–2008)
• Joined Novartis in 2003 as Head Finance Global Primary Care, and over the years held positions of
increasing responsibility within Finance
Mandates
• Represented Novartis on the board of GlaxoSmithKline Consumer Healthcare Holdings Ltd. (2015–2018)
Education
• Diploma degree in industrial engineering and economics, University of Karlsruhe, Germany
Robert (Rob) Kowalski
Chief People & Organization Ocer of Novartis since September 1, 2021 | Nationality: American | Year of birth: 1968
Professional experience
• Executive Vice President and Global Head of Regulatory Aairs (2018–2021), and US Head of Global
Drug Development (2009–2015 and 2017–2021), Novartis Pharmaceuticals Corporation, US
• Ad interim President, Novartis Corporation, US (2021)
• Ad interim Head of Global Drug Development and Chief Medical Ocer, Novartis AG,
Switzerland (2018)
• Senior Vice President and Head of Regulatory Aairs, Novartis Pharmaceuticals Corporation, US
(2009–2015 and 2017–2018)
• Senior Vice President and Head of Regulatory Aairs, Novartis Pharma AG, Switzerland (2015–2017)
• Global Head of Country Medical Development, Novartis Pharmaceuticals Corporation, US (2010–2011)
• Previously held regulatory leadership roles at Schering-Plough Corporation (now Merck) and Pharmacia
Corporation (now Pfizer)
Mandates
• Member of the advisory board, Industry Pharmacists Organization, US
Education
• Doctor of pharmacy, University of Wisconsin-Madison, US
• Bachelor of Science in pharmaceutical sciences, University of Wisconsin-Madison, US

Item 6. Directors, Senior Management and Employees
151
Steen Lang, Ph.D.
President, Operations since April 4, 2022 | Nationality: German/Swiss | Year of birth: 1967
Professional experience
• Global Head of Novartis Technical Operations (NTO), Switzerland (2017–April 2022)
• Global Head of Biologics Technical Development and Manufacturing, Novartis Technical Operations,
Switzerland (2015–2017)
• Global Head of Technical Research and Development, Novartis Pharmaceuticals, Switzerland (2009–2015)
• Joined Novartis in 1994 as Head of Laboratory in Research, and over the years held positions of
increasing responsibility within Pharmaceuticals Development
Mandates
• Board member, Bachem Holding AG, Switzerland
Education
• Doctorate in pharmaceutical technology, Swiss Federal Institute of Technology, Switzerland
• Master’s degree in pharmaceutical sciences, University of Heidelberg, Germany
Fiona H. Marshall, Ph.D.
President, Novartis Institutes for BioMedical Research (NIBR) since November 1, 2022 | Nationality: British |
Year of birth: 1964
Professional experience
• Senior vice president, head of discovery, preclinical and translational medicine, Merck & Co., US,
(2021–September 2022)
• Vice president, global head of neuroscience, Merck & Co., US (2019–2021)
• Vice president, head of UK discovery research, Merck & Co., UK (2018–2019)
• Executive vice president and chief scientific ocer, Sosei Heptares, UK (2015–2018)
• Chief scientific ocer and founder, Heptares Therapeutics, UK (2006–2018)
Mandates
• Member of the Scientific Advisory Board, SciLifeLab, Sweden
• Fellow, Royal Society, UK
• Honorary Fellow, Royal Society of Chemistry, UK
• Honorary Fellow, British Pharmacological Society, UK
• Fellow, UK Academy of Medical Sciences, UK
• Fellow, Royal Society of Biology, UK
Education
• PhD in Neuroscience, University of Cambridge, UK
• BSc in Biochemistry, University of Bath, UK
Klaus Moosmayer, Ph.D.
Chief Ethics, Risk & Compliance Ocer of Novartis since 2018 | Nationality: German | Year of birth: 1968
Professional experience
• Chief compliance ocer, Siemens AG, Germany (2014–2018)
• Chief counsel compliance, Siemens AG, Germany (2009–2013)
• Compliance operating ocer, Siemens AG, Germany (2007–2009)
Mandates
• Board member, SwissHoldings, the Swiss federation representing Swiss-based multinational companies,
Switzerland
• Member of the executive board, Business at OECD (BIAC), Paris
• Co-chair, B20 Integrity & Compliance Task Force under the G20 presidencies of Indonesia (2022), Italy
(2021), Saudi Arabia (2020), Argentina (2018), and Chair of the Task Force under the G20 presidency of
Germany (2017)
• Member of the advisory panel, Pharmaceutical Supply Chain Initiative, US
• Co-founder and board member, European Chief Compliance and Integrity Ocers’ Forum
• Chair of the Anti-Corruption Committee of the Business and Industry Advisory Committee (BIAC),
Organization for Economic Co-operation and Development (OECD), Paris (2013–2020)
Education
• First and second state examination in law, Germany
• Doctor of jurisprudence, University of Freiburg, Germany
Marie-France Tschudin
President, Innovative Medicines International & Chief Commercial Ocer since April 4, 2022 | Nationality: Swiss |
Year of birth: 1971
Professional experience
• President, Novartis Pharmaceuticals, Switzerland (2019–April 2022)
• President, Advanced Accelerator Applications, France (2019)
• Europe Region Head, Novartis Pharmaceuticals, Switzerland (2017–2019)
• Corporate vice president of hematology and oncology for Europe, the Middle East and Africa, Celgene
International, Switzerland (2014–2016)
• Regional vice president of northern Europe, Celgene International, Switzerland (2012–2014)
• General manager of Austria, Switzerland, the Czech Republic, Poland, Slovenia and Slovakia, Celgene
International, Switzerland (2009–2011)
• Country manager of Switzerland, Celgene International, Switzerland (2008–2009)
Mandates
• Board member, IMD Foundation, Switzerland
• Board member, AXA, France
• Board member, European Federation of Pharmaceutical Industries and Associations (EFPIA), Belgium
Education
• Master of Business Administration, IMD business school, Switzerland
• Bachelor of Science, Georgetown University, US

Item 6. Directors, Senior Management and Employees
152
Information and control systems
The Board’s information and control systems vis-à-vis
management include a steady flow of information from
senior management; monthly financial reports; a compre-
hensive and integrated risk management framework; and
the independent evaluation of our risk management and
internal control framework by the Internal Audit function
(see “Item 15. Controls and Procedures”).
Information from senior management
The Board ensures that it receives sucient information
from the Executive Committee through:
• Monthly CEO reporting (including detailed written
updates from each division and business unit head),
frequent communications from the CEO on current
developments, and a yearly presentation
• Executive Committee meeting minutes
• Regular meetings and teleconferences by the Board
and/or Board committees with the CEO and/or other
members of the Executive Committee (e.g., the CFO,
the Chief Legal Ocer, the Chief Ethics, Risk & Com-
pliance Ocer), and regular meetings and teleconfer-
ences with senior management (e.g., the Chief Audit
Ocer)
• Information from Executive Committee members or
other Novartis employees, and visits to Novartis sites
To get an outside view, the Board and/or Board commit-
tees occasionally invite external advisors (e.g., the inde-
pendent advisor of the Compensation Committee, the
external auditor) to attend a meeting and/or share their
observations about a specific topic.
Monthly financial reports
Novartis produces comprehensive, consolidated (unau-
dited) financial statements on a monthly basis for the
Group and its operating divisions. These are typically
available within 10 days after the end of the month, and
include the following:
• Consolidated income statement of the month and year to
date, in accordance with International Financial Report-
ing Standards (IFRS), as well as adjustments to arrive
at core results, as defined by Novartis (see “Item 5.
Operating and Financial Review and Prospects—Item
5.A Operating results—Non-IFRS measures as defined
by Novartis”). The IFRS and core figures are compared
with the prior-year period and targets in both USD and
on a constant currency basis.
• Supplementary data on a monthly and year-to-date
basis, such as free cash flow and earnings per share
on a USD basis
Management information related to the consolidated
income statements and free cash flow is made available
to Board members through the monthly CEO Report,
which includes an analysis of key deviations from the
prior year or target.
Prior to the release of each quarter’s results, the Board
receives the actual consolidated financial statement infor-
mation and an outlook of the full-year results in accor
-
dance with IFRS and core results (as defined by Novartis),
together with related commentary.
Annually, in the middle of the year, the Board approves
the Company’s strategic plan for the next three years. In
the fourth quarter of the year, the Board approves the
operating targets for the following year as well as the
financial targets for the following three-year period,
including a projected consolidated income statement in
USD prepared in accordance with IFRS and non-IFRS
measures as defined by Novartis (core results).
The Board does not have direct access to the Novartis
financial and management reporting systems but can, at
any time, request more detailed information.

Item 6. Directors, Senior Management and Employees
153
Risk management
Overview
At Novartis, our continued success depends on our abil-
ity to manage risk. Our Board has ultimate oversight of
the Enterprise Risk Management (ERM) system and reg-
ularly reviews the most significant risks and how these
risks are managed. As explained further below, the Board
is supported by its committees. Furthermore, our Internal
Audit function provides an independent evaluation of risk
management (see “—Item 6.C Board practices—Informa-
tion and control systems—Internal Audit”).
BOARD COMMITTEES
RISK COMMITTEE
• Oversees the risk management system and processes
• Reviews, together with management, the prioritization and handling
of risks, the risk portfolio, and actions implemented by management
• Performs deep dives into key risk areas and fosters a culture of
smart risk-taking
• Receives updates on cyber security on an annual basis
• Receives regular updates from designated risk owners as well as
the Chief Ethics, Risk & Compliance Ocer and/or the Head of Risk
& Resilience
AUDIT AND COMPLIANCE COMMITTEE
• Ensures that Internal Audit plans are aligned with key risks, and that
the function provides independent assurance and insights around
these risks
• Works closely with the Risk Committee to minimize gaps in
risk coverage
• Receives a semiannual presentation from the Chief Ethics, Risk &
Compliance Ocer
• Receives a quarterly presentation from the Chief Audit Ocer on
progress achieved in implementing the risk-based audit plan, and
key insights about audit and advisory activities
• Pays particular attention to financial risk
• Has closed sessions with the Chief Audit Ocer and, upon request,
with the Chief Ethics, Risk & Compliance Ocer
COMPENSATION COMMITTEE
• Works closely with the Risk Committee to ensure that the
compensation system does not lead to excessive risk-taking
(see “—Item 6.B Compensation—Compensation governance—
Risk management principles”)
EXECUTIVE COMMITTEE OF NOVARTIS
• Regularly assesses risks and fosters a culture of risk awareness,
in line with the Novartis Values and Behaviors and the Novartis
Code of Ethics
ETHICS, RISK & COMPLIANCE
• Governs the Novartis Code of Ethics
• Provides an integrated ERM framework (further described in the
following section)
• Governs the global compliance program within Novartis
• Administers the Enterprise Policy Management and global Internal
Controls framework
SENIOR LEADERS OF DIVISIONS, ORGANIZATIONAL UNITS
ANDGROUP FUNCTIONS, AT ALL LEVELS
• Provide appropriate risk management within their area of
responsibility
• Establish adequate risk prevention and mitigation strategies when
risk exposure is identified, including tracking progress and providing
resources for possible actions
• Assess emerging risks, trends and overall exposure as part of the
ERM process
Enterprise Risk Management framework
The Ethics, Risk & Compliance (ERC) function provides
an integrated ERM framework to obtain a holistic view of
Company risks and drive a culture of smart risk-taking.
Under the leadership of the Chief Ethics, Risk & Compli-
ance Ocer, the Risk & Resilience team is responsible
for the overall ERM process. This process covers, but is
not limited to, risks associated with:
• The research, development, manufacturing, marketing
and sales of products
• Finance, taxes, intellectual property, compliance with law
and regulations, security, product safety, human resources,
and health, safety and environmental protection
• Business objectives and strategies, including mergers
and acquisitions
• External factors such as the social, political and eco-
nomic environment
The ERM process continued to evolve in 2022. The Risk
& Resilience team conducted risk workshops and collab-
orated with all risk assurance and monitoring functions
to identify key risks across the Company. Each Novartis
unit organized a focused risk workshop at the leadership
team level. In parallel, risk workshops were held in the
top 11 countries (by revenue) and in certain focus mar-
kets. Once key risks were identified, mitigation action
plans were created to address them in an eective way.
The findings from these workshops were consolidated
into the Novartis Risk Compass, which enables senior
management, the Executive Committee and the Board
to focus discussions on key risks and more closely align
our corporate strategy with our risk exposure and ways
of working.
In 2022, we further matured our ERM framework
within the Novartis Risk & Resilience organization, devel-
oped additional risk management trainings, and inte-
grated other critical risk management functions (like
Third-Party Risk Management and Health, Safety and
Environment) into the Risk & Resilience department. Fur-
thermore, the Enterprise Policy & Internal Control team
is progressing as planned to create a holistic framework,
and the Central Monitoring Coordination team is expand-
ing its scope to ensure a harmonized and coordinated
monitoring process across the Company.
SpeakUp Oce
Our SpeakUp Oce provides a safe place for employ-
ees to report potential misconduct, including the option
to do so anonymously.
Global Security
Global Security proactively collects and shares threat
intelligence to protect Novartis from situations that may
compromise the safety of people, products and assets,
and/or the reputation of our organization. Global Security
protects patients from counterfeit products and, as part
of the SpeakUp process, performs fair and timely inves
-
tigations into high-risk cases of alleged internal miscon-
duct. It also provides personal security advice and sup-
port for Novartis executives and other employees with
the utmost discretion.

Internal Audit cycle methodology
includes:
3
Planning: Monitoring and information
gathering via continuous risk assess-
ment based on data analytics, busi-
ness interviews and quarterly calibra-
tion of the audit plan
3
Execution and Reporting: 63 engage-
ments delivered in 2022, all linked to
group risks, emerging topics and com-
pany-wide initiatives
3
Follow Up: Management is responsible
for resolving issues, supported by
Internal Audit to ensure timely closure
of observations
AUDITS
41
ADVISORIES
8
INTERNAL REVIEWS
14
INTERNAL AUDIT ACTIVITIES
Item 6. Directors, Senior Management and Employees
154
Internal Audit
The purpose of Internal Audit is to assist the Board and
the Executive Committee in discharging their governance
responsibilities by providing independent assurance and
advice on the eectiveness, eciency and adequacy of
processes and controls that support Novartis in achiev-
ing its objectives, managing its major risks, and ensuring
compliance with applicable policies, laws and regulations.
The Chief Audit Ocer reports administratively to
the CEO, and functionally to the chair of the Audit and
Compliance Committee (ACC). The Chief Audit Ocer
meets with the ACC at least once a quarter and confirms
the organizational independence of the Internal Audit
function to the ACC on an annual basis.
In 2022, our Internal Audit function executed a risk-
based audit plan and reported the results to the audited
units, the Executive Committee and the ACC. Audit find-
ings and action plans are stored and monitored in a sin-
gle location to enable ecient and eective follow-up.
The following outlines the number of audits, internal
reviews and advisories performed in 2022, and key meth-
odology steps when managing the Internal Audit cycle.
Internal Audit performed 85% of planned activities
(equating to 63 of 74 engagements) in 2022, conducted
under a hybrid model of engagement delivery, choos-
ing between remote and in-person auditing based on
the engagement scope and COVID-19 situation within
the audited entity.

Item 6. Directors, Senior Management and Employees
155
Auditors
Duration of the mandate
andtermsofoce
On behalf of the Board, the ACC selects and nominates
an independent auditor for election at the AGM. KPMG
commenced its auditing mandate for Novartis in 2022.
Richard Broadbelt, Auditor in charge, and Sara Burke,
Global Audit Partner, began serving in their roles in 2022.
The ACC together with KPMG will ensure that these part
-
ners are rotated at least every five years.
Auditing fees and additional fees
The ACC monitors and preapproves the fees paid to the
external auditor for all audit and non-audit services. It has
developed and approved a policy with clear guidelines
on the engagement of the independent auditor firm. This
policy is designed to help ensure that the independence
of the external auditor is maintained. It limits the scope of
services that the external auditor may provide to the Group,
stipulating certain permissible types of audit-related and
non-audit services, including tax services and other ser-
vices that have been preapproved by the ACC. The ACC
preapproves all other services on a case-by-case basis.
The external auditor is required to report periodically
to the ACC about the scope of the services it has pro-
vided to the Group and the fees for the services it has
performed to date. KPMG fees for professional services
related to the 12-month period ended December 31, 2022,
and PwC fees for professional services related to the
12-month period ended December 31, 2021, are as fol-
lows:
2022
2021
USD million
USD million
Audit services 22.5
22.2
Audit-related services 0.7
1.5
Tax services 1.2
0.1
Other services 0.0
1.4
Total 24.4
25.2
Audit services include work performed to issue opinions
on consolidated financial statements and parent company
financial statements of Novartis AG, to issue opinions related
to the eectiveness of the Group’s internal control over
financial reporting, and to issue reports on local statutory
financial statements. Also included are audit services that
generally can only be provided by the statutory auditor,
such as the audit of the Compensation Report, audits of
the adoption of new accounting policies, audits of infor-
mation systems and the related control environment, as
well as reviews of quarterly financial results.
Audit-related services include other assurance services
provided by the independent auditor but not restricted to
those that can only be provided by the statutory auditor.
They include services such as: audits of pension and
other employee benefit plans; audits in connection with
non-recurring transactions; contract audits of third-party
arrangements; corporate responsibility assurance; and
other audit-related services.
Tax services include tax compliance, assistance with
historical tax matters, and other tax-related services.
Other services in 2021 included procedures related
to corporate integrity agreements, benchmarking stud-
ies, and license fees for use of accounting and other
reporting guidance databases.
Information to the Board and the ACC
The ACC, acting on behalf of the Board, is responsible for
overseeing the activities of the external auditor. In 2022,
this committee held seven meetings. KPMG was invited to
all of these meetings to attend the discussions on audit-
ing matters and any other matters relevant to its audit.
The ACC recommended to the Board to approve the
audited consolidated financial statements and the separate
parent company financial statements of NovartisAG for the
year ended December 31, 2022. The Board proposed
the acceptance of these financial statements for approval
by the shareholders at the next AGM.
The ACC regularly evaluates the performance of the
external auditor and, based on this, once a year deter-
mines whether the external auditor should be proposed
to the shareholders for re-election. To assess the per-
formance of the external auditor, the ACC requests input
from management and holds private meetings with the
CFO and the Chief Audit Ocer and, if necessary, obtains
an independent external assessment. Criteria applied
for the performance assessment of the external auditor
include an evaluation of: its technical and operational
competence; its independence and objectivity; the suf-
ficiency of the resources it has employed; its focus on
areas of significant risk to Novartis; its willingness to
probe and challenge; its ability to provide eective, prac-
tical recommendations; and the openness and eective-
ness of its communications and coordination with the
ACC, the Internal Audit function and management.
Once a year, the Auditor in charge and the Global
Audit Partner report to the Board on the external audi-
tor’s activities during the current year, and on the audit
plan for the coming year.
On an annual basis, the external auditor provides the
ACC with written disclosures required by the US Public
Company Accounting Oversight Board, and the commit-
tee and the external auditor discuss the external audi-
tor’s independence from Novartis.

Item 6. Directors, Senior Management and Employees
156
Information policy
Novartis is committed to open and transparent commu-
nication with shareholders, investors, financial analysts,
customers, suppliers and other stakeholders. Novartis
disseminates information about material developments in
its businesses in a broad and timely manner that complies
with the rules of the SIX Swiss Exchange and the NYSE.
Communications
Novartis publishes this Annual Report to provide infor-
mation on the Group’s results and operations. Novartis
discloses financial results in accordance with IFRS on a
quarterly basis, and issues press releases from time to
time regarding business developments.
Novartis publishes press releases related to financial
results and material events to the US Securities and
Exchange Commission (SEC) via Form 6-K. An archive
containing annual reports, US SEC Form 20-F, quarterly
results releases and all related materials – including pre-
sentations and conference call webcasts – is available
at www.novartis.com/investors.
Novartis also publishes a Novartis in Society Inte-
grated Report, available at www.reporting.novartis.com,
which highlights progress on the Company’s strategic
priorities and describes how Novartis creates value for
diverse stakeholders. The Novartis in Society Integrated
Report has been prepared in alignment with the Inte-
grated Reporting Framework (part of the IFRS Founda-
tion), the Task Force on Climate-related Financial Dis-
closures (TCFD), the Sustainability Accounting Standards
Board (SASB) and the latest non-financial standards
issued by the Global Reporting Initiative (GRI). It also
contains our main disclosures against the Company’s
reporting requirement as a signatory of the United Nations
Global Compact.
The information on Board and Executive Committee
compensation is outlined in the Compensation Report (see
“—Item 6.B Compensation” in general, and for certain com
-
pensation information with respect to our Board that is
responsive to Item 6.C.2 of Form 20-F, see “—Item 6.B Com-
pensation—2022 Board compensation—Philosophy and
benchmarking”). Please also refer to articles 29-35 of the
Articles of Incorporation (www.novartis.com/investors/
company- overview/corporate-governance). There are no
change-of-control or ‘golden parachute’ clauses benefit-
ing Board members, Executive Committee members, or
other members of senior management. Employment con-
tracts with Executive Committee members are either for a
fixed term not exceeding one year or for an indefinite period
with a notice period not exceeding 12months, and do not
contain commissions for the acquisition or transfer of enter-
prises or severance payments. No loans or credits are
granted to Board and Executive Committee members.
Information contained in reports and releases issued
by Novartis is only correct and accurate at the time of
release. Novartis does not update past releases to reflect
subsequent events, and advises against relying on them
for current information.
Investor Relations
Investor Relations manages the Group’s interactions with
the international financial community. Several events are
held each year to provide institutional investors and analysts
with various opportunities to learn more about Novartis.
Investor Relations is based at the Group’s head quarters
in Basel. Part of the team is located in the US to coor-
dinate interaction with US investors. More information is
available at www.novartis.com/investors.
Website information
Topic Information
Share capital Articles of Incorporation of Novartis AG
www.novartis.com/investors/company-overview/corporate-governance
Novartis key share data
www.novartis.com/investors/share-data-analysis
Shareholder rights Articles of Incorporation of Novartis AG
www.novartis.com/investors/company-overview/corporate-governance
Annual General Meeting of Shareholders Annual General Meeting of Shareholders
www.novartis.com/investors/shareholder-information/annual-general-meeting
Board Regulations Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
Novartis code for senior financial ocers Novartis Code of Ethical Conduct for CEO and Senior Financial Ocers
www.novartis.com/investors/company-overview/corporate-governance
Novartis in Society Integrated Report Novartis in Society Integrated Report
www.reporting.novartis.com
Novartis financial data Novartis financial data
www.novartis.com/investors/financial-data
Press releases Press releases
www.novartis.com/news/news-archive?typemedia_release
Email service
www.novartis.com/news/stay-up-to-date
Additional information Novartis Investor Relations
(including Novartis investor event calendar, registered oce, www.novartis.com/investors
contact and email addresses, phone numbers, etc.)

Item 6. Directors, Senior Management and Employees
157
Quiet periods
According to our Global Insider Policy, employees who
have access to material non-public information on a reg-
ular basis are designated as Continuing Insiders and are
banned from trading in Novartis securities during quiet
periods. Limited exemptions for the expiry of options or
warrants within a quiet period apply. Until June 14, 2022,
our quarterly quiet periods commenced at the begin-
ning of the last trading day of each calendar quarter
and ended at the beginning of the first trading day after
the subsequent release of the quarterly and/or annual
results. Eective June 15, 2022, our quarterly quiet peri-
ods commence on the first trading day of each calen
-
dar quarter and end at the beginning of the first trading
day after the subsequent release of the quarterly and/
or annual results.
In 2022, the following quiet periods applied:
• December 30, 2021, until (and including) February 2,
2022
• March 31, 2022, until (and including) April 26, 2022
• July 1, 2022, until (and including) July 19, 2022
• October 1, 2022, until (and including) October 25, 2022

Item 6. Directors, Senior Management and Employees
158
6.D Employees
The table below sets forth the breakdown of the total year-end number of our full-time equivalent employees by
main category of activity and geographic area for the past three years.
For the year ended
December , Marketing and
Production and
Research and
General and
(full-time equivalents) sales
supply
development
Operations
administration
Tot a l
USA
Canada and Latin America
Europe
Asia/Africa/Australasia
Total
For the year ended
December , Marketing and
Production and
Research and
General and
(full-time equivalents) sales
supply
development
Operations
administration
Tot a l
USA
Canada and Latin America
Europe
Asia/Africa/Australasia
Tot al
For the year ended
December , Marketing and
Production and
Research and
General and
(full-time equivalents) sales
supply
development
Operations
administration
Tot a l
USA
Canada and Latin America
Europe
Asia/Africa/Australasia
Tot al
1
relates to full time equivalent employees (FTEs) from our Operations unit, excluding the Operations units’ production and supply FTEs
As of December 31, 2022, the total number of our full-
time equivalent employees decreased by 2 620 com
-
pared with December 31, 2021, mainly driven by the ini-
tiative announced in April 2022 to implement a new,
streamlined organizational model. For more information
about this new organizational structure, see “Item 4.
Information on the Company—Item 4.B Overview.”
A significant number of our employees are repre-
sented by unions or works councils. We have not expe-
rienced any material work stoppages in recent years, and
we consider our employee relations to be good.
6.E Share ownership
The information set forth under “Item 6. Directors, Senior
Management and Employees—Item 6.B Compensa-
tion—2021 Executive Committee compensation—Addi-
tional disclosures for the CEO and other Executive Com-
mittee members—Shares, ADRs and other equity rights
owned by Executive Committee members at Decem-
ber31, 2021” and under “Item 6. Directors, Senior Man-
agement and Employees—Item 6.B Compensation—2021
Board compensation—Additional disclosures—Shares,
ADRs and share options owned by Board members” is
incorporated by reference. For more information on our
equity-based participation plans, see the information set
forth under “Item 18. Financial Statements—Note26.
Equity-based participation plans for employees,” which
is incorporated by reference.

Item7. Major Shareholders and Related Party Transactions
159
Item7. Major Shareholders and Related Party
Transactions
7.A Major shareholders
Novartis shares are widely held. As of December31,
2022, Novartis had approximately 186 000 sharehold-
ers listed in the Share Register of Novartis, representing
approximately 67.0% of issued shares. Based on the
Novartis Share Register and excluding treasury shares,
approximately 48.4% of the shares registered by name
were held in Switzerland, and approximately 21.3% were
held in the US. Approximately 15.6% of the shares reg-
istered in the Share Register were held by individual
investors, while approximately 37.7% were held by legal
entities (excluding 7.7% of our share capital held as trea-
sury shares by Novartis AG or its fully owned subsidiar-
ies), and 46.7% were held by nominees, fiduciaries and
the ADS depositary. Due to a change in Swiss corporate
law, as of January 1, 2023, Novartis ordinary shares held
by certain Swiss foundations controlled by Novartis
(Foundation Shares) no longer carry the right to vote. As
a result, in the future these Foundation Shares will be
excluded from the calculation of the shares registered
in the Share Register in the same way, as described
above, that our treasury shares are excluded. This will
impact some of the percentage holdings reported in this
Item 7.A in future Form 20-F filings by Novartis.
Based on the Share Register, we believe that we are
not directly or indirectly owned or controlled by another
corporation or government, or by any other natural or
legal persons. There are no arrangements that may result
in a change of control.
The tables below set forth information with respect
to our major shareholders according to the Share Reg-
ister as of December 31, 2022, excluding 7.7% of our
share capital held as treasury shares by Novartis AG or
its fully owned subsidiaries. The following registered
shareholders (including nominees and the ADS deposi-
tary) held more than 2% of the total share capital of
Novartis with the right to vote all their Novartis shares
based on an exemption granted by the Board of Direc-
tors:
% of respective share capital beneficially owned
as of:
Ordinary shares
beneficially owned as of
Dec , Dec , Dec , Dec ,
Shareholders registered for their own account:
Emasan AG, Basel, Switzerland
UBS Fund Management (Switzerland) AG, Basel, Switzerland
Credit Suisse Funds AG, Zurich, Switzerland
% of respective share capital held as of:
Ordinary shares
held as of
Dec ,
Dec ,
Dec ,
Dec ,
Shareholders registered as nominees:
Chase Nominees Ltd., London, England
Nortrust Nominees Ltd., London, England
The Bank of New York Mellon, New York, NY
Through The Bank of New York Mellon, Everett, MA
Through The Bank of New York Mellon, New York, NY
Through The Bank of New York Mellon, SA/NV, Brussels, Belgium
Shareholder acting as American Depositary Share (ADS) depositary:
JPMorgan Chase Bank, N.A., New York, NY
According to a disclosure notification filed with Novartis
AG, Norges Bank (Central Bank of Norway), Oslo, Nor-
way, held 2.3% of the share capital of Novartis AG, or 54
667 792 shares, as of December31, 2022, but was not
registered in the Share Register as of December31,
2022. Provided that these shares are registered in the
Share Register on the record date of the Annual General
Meeting, Norges Bank will have full voting rights for all
of these shares.
According to a disclosure notification filed with
Novartis AG and the SIX Swiss Exchange, Black
-
Rock,Inc., NewYork, NY, held between 5% and 10%, but
was registered with less than 2% of the share capital of

Item7. Major Shareholders and Related Party Transactions
160
Novartis AG in the Share Register as of December31,
2022.
As of December31, 2022, no other shareholder was
registered as owner of more than 2% of the registered
share capital.
The Articles of Incorporation provide that no share-
holder shall be registered with the right to vote shares
comprising more than 2% of the registered share
capital. The Board of Directors may, upon request, grant
an exemption from this restriction. Considerations
include whether the shareholder supports the Novartis
goal of creating sustainable value and has a long-term
investment horizon. Exemptions are in force for the reg-
istered major shareholders as described above. Novartis
has not entered into any agreement with any shareholder
regarding the voting or holding of Novartis shares.
7.B Related party transactions
The information set forth under “Item 18. Financial Statements—Note27. Transactions with related parties” is incor-
porated by reference.
7.C Interests of experts and counsel
Not applicable.

Item8. Financial Information
161
Item8. Financial Information
8.A Consolidated statements and other financial
information
See “Item18. Financial Statements.”
Dividend policy
Subject to the dividend policy described below, our
Board of Directors expects to recommend the payment
of a dividend in respect of each financial year. If approved
by our shareholders at the relevant annual shareholders’
meeting, the dividends will be payable shortly following
such approval. Any shareholder who purchases our
shares before the ex-dividend date and holds the shares
until that date shall be deemed to be entitled to receive
the dividends approved at that meeting. Dividends are
reflected in our financial statements in the year in which
they are approved by our shareholders.
Our dividend policy is to pay a growing annual divi-
dend in Swiss francs per share. This policy is subject to
our financial conditions and outlook at the time, the
results of our operations, and other factors.
The Board will propose a dividend of CHF 3.20 per
share to the shareholders for approval at the Annual
General Meeting to be held on March 7, 2023. Because
we pay dividends in Swiss francs, exchange rate fluctu-
ations will aect the US dollar amounts received by hold-
ers of ADRs. For the amount of dividends we paid in the
past three years, see “Item 18. Financial Statements—
Note 18—Equity.”
Disclosure pursuant to Section219 of the Iran
Threat Reductionand Syria Human Rights Act
(ITRA)
At Novartis, our purpose is to reimagine medicine to
improve and extend people’s lives, regardless of where
they live. This includes the compliant sale of medicines
and other healthcare products worldwide. To help us ful-
fill this mission, we have for many years maintained two
representative oces located in Iran.
As of October18, 2010, a non-US aliate within our
Innovative Medicines Division entered into a non-bind-
ing Memorandum of Understanding (MoU) with the Min-
istry of Health and Medical Education of the Islamic
Republic of Iran. Pursuant to the MoU, the Iranian Minis-
try of Health acknowledges certain benefits that may
apply to sales of certain Innovative Medicines Division
medicines by third-party distributors in Iran. These
include fast-track registration, market exclusivity,
end-user subsidies, and exemptions from customs tar-
is. Novartis receives no payments from the Iranian Min-
istry of Health under the MoU, and the MoU creates no
obligations on the part of either Novartis or the Iranian
Ministry of Health.
From time to time, including in 2022, non-US aliates
in our Innovative Medicines and Sandoz Divisions made
payments to government entities in Iran related to pat-
ents, trademarks, exit fees and other transactions ordi-
narily incident to travel by doctors and other medical pro-
fessionals resident in Iran to attend conferences or other
events outside Iran.
From time to time, including in 2022, non-US aliates
in our Innovative Medicines and Sandoz Divisions enter
into agreements with hospitals, research institutes, med-
ical associations and universities in Iran to provide grants
and sponsor congresses, seminars and symposia, and
with doctors and other healthcare professionals for con-
sulting services, including participation in advisory
boards and investigator services for observational
(non-interventional) studies. Some hospitals and
research institutes are owned or controlled by the gov-
ernment of Iran, and some doctors and healthcare pro-
fessionals are employed by hospitals that may be public
or government-owned.
Because our Innovative Medicines and Sandoz Divi-
sions have operations in Iran, including employees, they
obtain services and have other dealings incidental to
their activities in that country, including paying taxes and
salaries either directly or indirectly through a service pro-
vider, and obtaining oce rentals, insurance, electricity,
water and telecommunications services, oce and sim-
ilar supplies, and customs-related services from Iranian
companies that may be owned or controlled by the gov-
ernment of Iran. In addition, from time to time, represen-
tatives of our non-US aliates participate in meetings
with Iranian ocials to discuss issues relevant to our
business and the pharmaceutical industry.
Non-US aliates in our Innovative Medicines and
Sandoz Divisions maintain local accounts at banks that
are, as of November 5, 2018, on the Specially Designated
Nationals and Blocked Persons List (SDN List). These
non-US aliates make local transactions for employee
payroll and local vendor payment purposes. These trans-
actions are conducted for the purpose of facilitating the
provision of medicine to Iran, in line with the humanitar-
ian exceptions contained in Section 11 of Executive Order
13902 and other applicable sanctions legal authorities.
No transactions are made with an Iranian financial insti-
tution designated on the SDN List in connection with
Iran’s support for international terrorism or proliferation
of weapons of mass destruction.

Item8. Financial Information
162
8.B Significant changes
None.

Item9. The Oer and Listing
163
Item9. The Oer and Listing
9.A Oer and listing details
Our shares are listed in Switzerland on the SIX Swiss
Exchange (SIX).
ADSs, each representing one share, have been avail-
able in the US through an ADR program since Decem-
ber 1996. This program was established pursuant to a
deposit agreement that we entered into with JPMorgan
Chase Bank, N.A., as depositary (“Deposit Agreement”).
Our ADRs have been listed on the NYSE since May 2000
and are traded under the symbol NVS.
The depositary has informed us that as of January
25, 2023, there were 220 million ADRs outstanding, each
representing one Novartis share (approximately 9% of
total Novartis shares issued). On January 25, 2023, the
closing price was CHF 85.30 per share on the SIX, and
USD 92.81 per ADR on the NYSE.
9.B Plan of distribution
Not applicable.
9.C Markets
See “—Item9.A Oer and listing details.”
9.D Selling shareholders
Not applicable.
9.E Dilution
Not applicable.
9.F Expenses of the issue
Not applicable.

Item10. Additional Information
164
Item10. Additional Information
10.A Share capital
Not applicable.
10.B Memorandum and articles of association
The following is a non-exhaustive summary of certain
provisions of our Articles of Incorporation (“Articles”);
our Regulations of the Board, the Board Committees and
the Executive Committee (“Board Regulations”); and
Swiss law, particularly the Swiss Code of Obligations
(“Swiss CO”), and is qualified in its entirety by reference
to the Articles and the Board Regulations, which are an
exhibit to this Form20-F, and to Swiss law.
10.B.1 Company purpose
Novartis AG is registered in the commercial register of
the canton of Basel-Stadt, Switzerland, under number
CHE-103.867.266. Our business purpose, as stated in
Article2 of the Articles, is to hold interests in enterprises
in the area of healthcare or nutrition. We may also hold
interests in enterprises in the areas of biology, chemis-
try, physics, information technology or related areas. We
may acquire, mortgage, liquidate or sell real estate and
intellectual property rights in Switzerland or abroad. In
pursuing our business purpose, we strive to create sus-
tainable value.
10.B.2 Directors
According to our Articles, the Board of Directors
(“Board”) consists of a minimum of eight and a maximum
of 16 members. The members of the Board (including the
Board Chair) are elected individually by the General
Meeting of Shareholders (“General Meeting”) for a one-
year term of oce lasting until completion of the next
Annual General Meeting of Shareholders (“AGM”).
(a) A Board resolution requires the armative majority
of the votes cast. According to our Board Regulations,
a member of our Board (“Director”) may not partici-
pate in decisions and resolutions on matters that
aect, or reasonably might aect, the Director’s inter-
ests or the interests of a person close to the Direc-
tor.
(b) Compensation of the Directors is subject to the
approval of the aggregate amounts of such compen-
sation by a shareholders’ resolution under the Ordi-
nance against Excessive Compensation in Public
Companies of the Swiss Federal Council.
(c) The Articles prohibit the granting of loans or credits
to Directors.
(d) The Articles provide that a Director shall not serve on
the Board for more than 12 years. The Board may,
under certain circumstances and if deemed in the
best interests of the Company, recommend excep-
tions to this rule to the General Meeting.
(e) Our Directors are not required to be shareholders at
the time of the election by the General Meeting. How-
ever, according to our share ownership guidelines,
the Board Chair is required to own a minimum of 30
000 Novartis AG shares, and other Directors are
required to own at least 5 000 Novartis AG shares
within five years after joining the Board, to ensure
their interests are aligned with those of our share-
holders.
10.B.3 Shareholder rights
Because Novartis AG has only one class of registered
shares, the following information applies to all sharehold-
ers.
(a) Under the Swiss CO, we may only pay dividends out
of balance sheet profits or out of distributable
reserves. In any event, under the Swiss CO, while the
Board may propose that a dividend be paid, we may
only pay dividends upon shareholders’ approval at a
General Meeting. Furthermore, the Swiss CO requires
us to accrue general legal reserves under certain cir-
cumstances so long as these reserves amount to less
than 20% of our registered share capital, and Swiss
law and the Articles permit us to accrue additional
reserves beyond the statutory reserves. Our auditors
must confirm that the dividend proposal of our Board
conforms with the Swiss CO and the Articles. Our
Board expects to recommend the payment of a divi-
dend in respect of each financial year. See “Item 6.
Directors, Senior Management and Employees—Item
6.C Board Practices—Capital Structure—Limitation
on transferability—Per-share information” and
“Item 8. Financial Information—Item 8.A. Consoli
-
dated statements and other financial information—
Dividend policy.”
Dividends are usually due and payable shortly after
the shareholders have passed a resolution approving
the payment. Dividends that have not been claimed
within five years after the due date revert to us and are
allocated to our general reserves. For information
about deduction of the withholding tax or other duties
from dividend payments, see “—Item10.E Taxation.”

Item10. Additional Information
165
(b) Each share is entitled to one vote at a General Meet-
ing. Voting rights may only be exercised for shares
registered with the right to vote on the record date
for the applicable General Meeting. In order to do so,
the shareholder must filea share registration form
with us, setting forth the shareholder’s name, address
and citizenship (or, in the case of a legal entity, its reg-
istered oce). If the shareholder has not timely reg-
istered its shares, then the shareholder may not vote
at, or participate in, a General Meeting.
To vote its shares, the shareholder must also
explicitly declare that it has acquired the shares in its
own name and for its own account. If the shareholder
refuses to make such a declaration, the shares may
not be voted unless the Board recognizes such share-
holder as a nominee.
The Articles provide that no shareholder shall be
registered with the right to vote shares comprising
more than 2% of the registered share capital. The
Board may, upon request, grant an exemption from
this restriction. Considerations include whether the
shareholder supports our goal of creating sustainable
value and has a long-term investment horizon. Fur-
thermore, the Articles provide that no nominee shall
be registered with the right to vote shares compris-
ing more than 0.5% of the registered share capital.
The Board may, upon request, grant an exemption
from this restriction if the nominee discloses the
names, addresses, and number of shares of the per-
sons for whose account it holds 0.5% or more of the
registered share capital. The same restrictions indi-
rectly apply to ADR holders. We have in the past
granted exemptions from the 2% rule for sharehold-
ers and the 0.5% rule for nominees.
For purposes of the 2% rule for shareholders and
the 0.5% rule for nominees, groups of companies and
groups of shareholders acting in concert are consid-
ered to be one shareholder. These rules also apply to
shares acquired or subscribed by the exercise of sub-
scription, option or conversion rights.
After hearing the registered shareholder or nom-
inee, the Board may cancel, with retroactive eect as
of the date of registration, the registration of the
shareholders if the registration was eected based
on false information.
Registration restrictions in the Articles may only
be removed upon a resolution carrying a two-thirds
majority of the votes represented at a General Meet-
ing.
Except as noted below, shareholders’ resolutions
require the approval of an absolute majority of the
votes present at a General Meeting. As a result,
abstentions have the eect of votes against such res-
olutions. Some examples of shareholders’ resolutions
requiring a vote by such “absolute majority of the
votes” are:
• Adoption and amendment of the Articles
• Election and removal of the Board Chair, the Board
and Compensation Committee members, the Inde-
pendent Proxy and the external auditor
• Approval of the management report and of the con-
solidated financial statements
• Approval of the financial statements of Novartis AG,
and decision on the appropriation of available earn-
ings shown on the balance sheet, including divi-
dends, if any
• Approval of the maximum aggregate compensation
of the Board (from an AGM until the next AGM) and
of the Executive Committee (for the financial year
following the AGM)
• Discharge of Board and Executive Committee
members from liability for matters disclosed to the
General Meeting
• Decision on other matters that are reserved by law
or by the Articles (e.g., advisory vote on the Com-
pensation Report) to the General Meeting
According to the Articles and Swiss law, the fol-
lowing matters require the approval of a “superma-
jority” of at least two-thirds of the votes present at a
General Meeting:
• Alteration of the purpose of Novartis AG
• Creation of shares with increased voting powers
• Implementation of restrictions on the transfer of
registe red shares, and the removal of such restric-
tions
• Authorized or conditional increase of the share cap-
ital
• Increase of the share capital out of equity, by con-
tribution in kind, for the purpose of an acquisition
of property or the grant of special rights
• Restriction or cancellation of subscription rights
• Change of the registered oce of NovartisAG
• Dissolution of Novartis AG
In addition, the law provides for a qualified major-
ity for other resolutions, such as a merger or demerger.
Our shareholders are required to annually elect
all Directors (including the Board Chair), the Compen-
sation Committee members, the external auditor and
the Independent Proxy. The Articles do not provide
for cumulative voting of shares.
At a General Meeting, shareholders can be repre-
sented by a proxy, which must either be the sharehold-
er’s legal representative, another shareholder with the
right to vote, or the Independent Proxy. Votes are taken
either by a show of hands or by electronic voting,
unless the General Meeting resolves to have a ballot
or where a ballot is ordered by the chair of the meet-
ing. ADSs, each representing one Novartis AG share
and evidenced by ADRs, are issued by our depositary
JPMorgan Chase Bank, N.A., New York, and not by
us. The ADR is vested with rights defined and enu-
merated in the Deposit Agreement (such as the rights
to vote, to receive a dividend and to receive a share
of Novartis AG in exchange for a certain number of

Item10. Additional Information
166
ADRs). The enumeration of rights, including any lim-
itations on those rights in the Deposit Agreement, is
final. There are no other rights given to the ADR hold-
ers. Only the ADS depositary, holding our shares
underlying the ADRs, is registered as shareholder in
our share register. An ADR is not a Novartis AG share
and an ADR holder is not a Novartis AG shareholder.
The Deposit Agreement between our depositary,
the ADR holder and us has granted certain indirect
rights to vote to the ADR holders. ADR holders may
not attend a General Meeting in person. ADR holders
exercise their voting rights by instructing JPMorgan
Chase Bank, N.A., our depositary, to exercise the vot-
ing rights attached to the registered shares underly-
ing the ADRs. Each ADR represents one Novartis AG
share. JPMorgan Chase Bank, N.A., exercises the vot-
ing rights for registered shares underlying ADRs for
which no voting instructions have been given by pro-
viding a discretionary proxy to an uninstructed inde-
pendent designee. Such designee has to be a share-
holder of Novartis AG. The same voting restrictions
apply to ADR holders as to those holding Novartis AG
shares (i.e., the right to vote up to 2% of the Novartis
AG registered share capital – unless otherwise
granted an exemption by the Board – and the disclo-
sure requirement for nominees).
(c) Shareholders have the right to allocate the profit
shown on our balance sheet and to distribute divi-
dends by vote taken at the General Meeting, subject
to the legal requirements described in “Item10.B.3(a)
Shareholder rights.”
(d) Under the Swiss CO, any surplus arising out of a liq-
uidation of Novartis AG (i.e., after the settlement of all
claims of all creditors) would be distributed to the
shareholders in proportion to the paid-in nominal
value of their shares.
(e) The Swiss CO limits a corporation’s ability to hold or
repurchase its own shares. We and our subsidiaries
may only repurchase shares if we have sucient
freely disposable equity in the amount of the pur
-
chase price of the acquired shares. The aggregate
nominal value of all Novartis AG shares held by us and
our subsidiaries may not exceed 10% of our regis-
tered share capital. However, it is accepted that a
Swiss corporation may repurchase its own shares
beyond the statutory limit of 10% if the repurchased
shares are clearly earmarked for cancellation. In addi-
tion, we are required to recognize a negative position,
or if our subsidiaries acquire our shares, to create a
special reserve on our balance sheet in the amount
of the purchase price of the acquired shares. Repur-
chased shares held by us or our subsidiaries do not
carry any rights to vote at a General Meeting, but are
entitled to the economic benefits generally con-
nected with the shares. The definition of subsidiaries,
and therefore, treasury shares, for purposes of the
above-described reserves requirement and voting
restrictions, diers from the definition of subsidiaries
for purposes of consolidation in our consolidated
financial statements. The definition in the consoli
-
dated financial statements requires consolidation for
financial reporting purposes of special purpose enti-
ties in instances where we have the power to govern
the financial and operating policies of the entity so as
to obtain benefits from its activities. Therefore, our
consolidated financial statements include special
purpose entities, mainly foundations, which do not
qualify as subsidiaries subject to the reserve require-
ments and voting restrictions of the Swiss CO because
we do not hold a majority participation in these spe-
cial purpose entities. Accordingly, no reserve require-
ments apply to shares held by such special purpose
entities, and such entities are not restricted from inde-
pendently voting their shares.
Under the Swiss CO, we may not cancel treasury
shares without the approval of a capital reduction by
our shareholders.
(f) Not applicable.
(g) Since all of our issued and outstanding shares have
been fully paid in, our shareholders are not obliged to
make further contributions with respect to their
shares.
(h) See “—Item 10.B.3(b) Shareholder rights” and “—
Item10.B.7 Change in control.”
10.B.4 Changes to shareholder rights
Under the Swiss CO, we may not issue new shares with-
out the prior approval of a capital increase by our share-
holders. If a capital increase is approved, then our share-
holders would generally have certain pre-emptive rights
to obtain newly issued shares in an amount proportional
to the nominal value of the shares they already hold.
These pre-emptive rights could be excluded in certain
limited circumstances with the approval of a resolution
adopted at a General Meeting by a supermajority of
two-thirds of the votes. In addition, we may not create
shares with increased voting powers or place restrictions
on the transfer of registered shares without the approval
of a resolution adopted at a General Meeting by a super-
majority of votes. In addition, see “—Item10.B.3(b) Share-
holder rights” with regard to the Board’s ability to cancel
the registration of shares under limited circumstances.
10.B.5 Shareholder meetings
Under the Swiss CO and the Articles, we must hold an
AGM within six months after the end of our financial year.
A General Meeting may be convened by the Board or, if
necessary, by the external auditor. The Board is further
required to convene an extraordinary General Meeting
if so resolved by a General Meeting, or if so requested
by shareholders representing at least 10% of the share
capital, specifying the items for the agenda and their
proposals. Shareholders representing shares with an
aggregate nominal value of at least CHF1000000 may
request that an item be included in a General Meeting
agenda. A General Meeting is convened by publishing a
notice in the Swiss Ocial Gazette of Commerce
(Schweizerisches Handelsamtsblatt) at least 20days
prior to such meeting. Shareholders may also be informed
by mail. Neither the Swiss CO nor the Articles require a
quorum for a General Meeting. In addition, see “—
Item10.B.3(b) Shareholder rights” regarding conditions
for exercising a shareholder’s right to vote at a General
Meeting.

Item10. Additional Information
167
10.B.6 Limitations
There are no limitations under the Swiss CO or our Arti-
cles on the right of non-Swiss residents or nationals to
own or vote shares other than the restrictions applica-
ble to all shareholders. But see “—Item10.B.3(b) Share-
holder rights” regarding conditions for exercising an ADR
holder’s right to vote at a shareholder meeting.
10.B.7 Change in control
The Articles and the Board Regulations contain no pro-
vision that would have an eect of delaying, deferring or
preventing a change in control of Novartis AG and that
would operate only with respect to a merger, acquisition
or corporate restructuring involving us or any of our sub-
sidiaries.
According to the Swiss Merger Act, shareholders
may pass a resolution to merge with another corpora-
tion at any time. Such a resolution would require the con-
sent of at least two-thirds of all votes present at the nec-
essary General Meeting.
Under the Swiss Financial Market Infrastructure Act,
shareholders and groups of shareholders acting in con-
cert who acquire more than 33 1/3% of our shares would
be under an obligation to make an oer to acquire all
remaining Novartis AG shares. Novartis AG has neither
opted out from the mandatory takeover oer obligation
nor opted to increase the threshold for mandatory take-
over oers in its Articles.
10.B.8 Disclosure of shareholdings
Under the Swiss Financial Market Infrastructure Act, per-
sons who directly, indirectly or in concert with other
parties acquire or dispose of our shares or purchase or
sale rights relating to our shares are required to notify
us and the SIX of the level of their holdings whenever
such holdings reach, exceed or fall below certain thresh-
olds – 3%, 5%, 10%, 15%, 20%, 25%, 33 1/3%, 50% and
66 2/3% – of the voting rights represented by our share
capital (whether exercisable or not). This also applies to
anyone who has discretionary power to exercise voting
rights associated with our shares. Following receipt of
such notification, we are required to inform the public by
publishing the information via the electronic publication
platform operated by the SIX.
An additional disclosure obligation exists under the
Swiss CO that requires us to disclose, once a year in the
notes to the financial statements published in our Annual
Report, the identity of all of our shareholders (or related
groups of shareholders) who have been granted exemp-
tion entitling them to vote more than 2% of our registered
share capital, as described in “—Item10.B.3(b) Share-
holder rights.”
10.B.9 Dierences in the law
See the references to Swiss law throughout this “—
Item10.B Memorandum and articles of association.”
10.B.10 Changes in capital
The requirements of the Articles regarding changes in
capital are not more stringent than the requirements of
Swiss law.
10.C Material contracts
Acquisition of The Medicines
Company
On November 23, 2019, we entered into an Agreement
and Plan of Merger (the “Merger Agreement”) with
US-based pharmaceutical company The Medicines
Company. Pursuant to the Merger Agreement, on Decem-
ber 5, 2019, Novartis, through a subsidiary, commenced
a tender oer to acquire all outstanding shares of The
Medicines Company for USD 85 per share, or a total con-
sideration of approximately USD 9.6 billion in cash on a
fully diluted basis. The tender oer expired on January
3, 2020, and on January 6, 2020, the acquiring subsid-
iary merged with and into The Medicines Company,
resulting in The Medicines Company becoming an
indirect wholly owned subsidiary of Novartis. This merger
broadens our cardiovascular portfolio by adding incli-
siran, an investigational cholesterol-lowering therapy.
Divestment of Roche shares
On November 3, 2021, we entered into a Share Repur-
chase Agreement with Roche under which we agreed to
sell 53.3 million (approximately 33%) of Roche bearer
shares in a bilateral transaction to Roche for a total con-
sideration of USD 20.7 billion. The transaction was
approved by the shareholders of Roche on Novem
-
ber26,2021, and closed on December 6, 2021.

Item10. Additional Information
168
10.D Exchange controls
There are no Swiss governmental laws, decrees or reg-
ulations that aect – in a manner material to Novartis AG
– the export or import of capital, including the availabil-
ity of cash and cash equivalents for use by Novartis or
any foreign exchange controls that aect the remittance
of dividends, interest or other payments to non-residents
or non-citizens of Switzerland who hold Novartis AG
securities.
10.E Taxation
The taxation discussion set forth below is intended only
as a descriptive summary and does not purport to be a
complete analysis or listing of all potential tax eects rel-
evant to the ownership or disposition of our shares or
ADRs. The statements of US and Swiss tax laws set forth
below are based on the laws and regulations in force as
of the date of this 20-F – including the current Conven-
tion Between the US and the Swiss Confederation for
the Avoidance of Double Taxation with Respect to Taxes
on Income, entered into force on December19, 1997 (“the
Treaty”); the US Internal Revenue Code of 1986, as
amended (“the Code”); Treasury regulations; rulings; judi-
cial decisions; and administrative pronouncements – and
may be subject to any changes in US and Swiss law, and
in any double taxation convention or treaty between the
US and Switzerland occurring after that date, which
changes may have retroactive eect.
Swiss taxation
Swiss residents
Withholding Tax on dividends and distributions. Divi-
dends that we pay and similar cash or in-kind distribu-
tions that we may make to a holder of shares or ADRs
(including distributions of liquidation proceeds in excess
of the nominal value, stock dividends and, under certain
circumstances, proceeds from repurchases of shares
by us in excess of the nominal value) are generally sub-
ject to a Swiss federal withholding tax (“the Withholding
Tax”) at a current rate of 35%. Under certain circum-
stances, distributions out of capital contribution reserves
made by shareholders after December 31, 1996, are
exempt from the Withholding Tax. We are required to
withhold Withholding Tax due from the gross distribution
and to pay the Withholding Tax to the Swiss Federal Tax
Administration. The Withholding Tax is refundable in full
to Swiss tax residents who are the beneficial owners of
the taxable distribution at the time it is resolved and duly
report the gross distribution received on their personal
tax return or in their financial statements for tax pur-
poses, as the case may be.
Income tax on dividends. A Swiss tax resident who
receives dividends and similar distributions (including
stock dividends and liquidation surplus) on shares or
ADRs is required to include such amounts in the share-
holder’s personal income tax return. However,
distributions out of qualified capital contribution reserves
are not subject to income tax. A corporate shareholder
may claim substantial relief from taxation of dividends
and similar distributions received if the shares held rep-
resent a fair market value of at least CHF1million.
Capital gains tax upon disposal of shares. Under current
Swiss tax law, the gain realized on shares held by a Swiss
resident who holds shares or ADRs as part of his private
property is generally not subject to any federal, cantonal
or municipal income taxation on gains realized on the
sale or other disposal of shares or ADRs. However, gains
realized upon a repurchase of shares by us may be char-
acterized as taxable dividend income if certain condi-
tions are met. Book gains realized on shares or ADRs
held by a Swiss corporate entity or by a Swiss resident
individual as part of the shareholder’s business property
are, in general, included in the taxable income of such
person. However, the Federal Law on the Direct Federal
Tax of December14, 1990, and several cantonal laws on
direct cantonal taxes provide for exceptions for Swiss
corporate entities holding more than 10% of our voting
stock for more than one year.
Residents of other countries
Recipients of dividends and similar distributions on our
shares who are neither residents of Switzerland for tax
purposes nor holding shares as part of a business con-
ducted through a permanent establishment situated in
Switzerland (“Non-Resident Holders”) are not subject to
Swiss income taxes in respect of such distributions.
Moreover, gains realized by such recipients upon the dis-
posal of shares are not subject to Swiss income taxes.
Non-Resident Holders of shares are, however, sub-
ject to the Withholding Tax on dividends and similar dis-
tributions mentioned above and, under certain circum-
stances, to the Stamp Duty described below. Such
Non-Resident Holders may be entitled to a partial refund
of the Withholding Tax if the country in which they reside
has entered into a bilateral treaty for the avoidance of
double taxation with Switzerland. Non-Resident Holders
should be aware that the procedures for claiming treaty
refunds (and the time frame required for obtaining a
refund) may dier from country to country. Non-Resident
Holders should consult their own tax advisors regarding
receipt, ownership, purchase, sale or other dispositions
of shares or ADRs, and the procedures for claiming a
refund of the Withholding Tax.

Item10. Additional Information
169
As of January1, 2023, Switzerland has entered into bilateral treaties for the avoidance of double taxation with
respect to income taxes with the following countries, whereby a part of the above-mentioned Withholding Tax may
be refunded (subject to the limitations set forth in such treaties):
Albania
Algeria
Argentina
Armenia
Australia
Austria
Azerbaijan
Bahrain
Bangladesh
Belarus
Belgium
Brazil
Bulgaria
Canada
Chile
China
Colombia
Croatia
Cyprus
Czech Republic
Denmark
Ecuador
Egypt
Estonia
Finland
France
Georgia
Germany
Ghana
Greece
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Republic of Ireland
Israel
Italy
Ivory Coast
Jamaica
Japan
Kazakhstan
Republic of Korea
(South Korea)
Kosovo
Kuwait
Kyrgyzstan
Latvia
Liechtenstein
Lithuania
Luxembourg
Malaysia
Malta
Mexico
Moldova
Mongolia
Montenegro
Morocco
Netherlands
New Zealand
North Macedonia
Norway
Oman
Pakistan
Peru
Philippines
Poland
Portugal
Qatar
Romania
Russia
Saudi Arabia
Serbia
Singapore
Slovak Republic
Slovenia
South Africa
Spain
Sri Lanka
Sweden
Taiwan
Tajikistan
Thailand
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Ukraine
United Arab Emirates
United Kingdom
United States of America
Uruguay
Uzbekistan
Venezuela
Vietnam
Zambia
Tax treaty negotiations are underway, or have been conducted, with Angola, Bosnia and Herzegovina, Cameroon,
Costa Rica, Ethiopia, Jordan, Kenya, Libya, Nigeria, Rwanda, Senegal, Syria and Zimbabwe. Tax treaty negotiations
between Switzerland and some of the countries listed in the immediately preceding sentence have been ongoing
for an extended period of time, and we are not certain when or if such negotiations will be completed, and when or
if the corresponding treaties will come into eect.
A Non-Resident Holder of shares or ADRs will not be lia-
ble for any Swiss taxes other than the Withholding Tax
described above and, if the transfer occurs through or
with a Swiss bank or other Swiss securities dealer, the
Stamp Duty described below. If, however, the shares or
ADRs of Non-Resident Holders can be attributed to a
permanent establishment or a fixed place of business
maintained by such person within Switzerland during the
relevant tax year, the shares or ADRs may be subject to
Swiss income taxes in respect of income and gains real-
ized on the shares or ADRs, and such person may qual-
ify for a full refund of the Withholding Tax based on Swiss
tax law.
Residents of the US. A Non-Resident Holder who is a
resident of the US for purposes of the Treaty is eligible
for a reduced rate of tax on dividends equal to 15% of
the dividend, provided that such holder (i)qualifies for
benefits under the Treaty, (ii)is not a company (or, if it is
a company, such company directly holds less than 10%
of our voting stock), and (iii)does not conduct business
through a permanent establishment or fixed base in Swit-
zerland to which the shares or ADRs are attributable.
Such an eligible holder must apply for a refund of the
amount of the Withholding Tax in excess of the 15%
Treaty rate. A Non-Resident Holder who is a resident of
the US for purposes of the Treaty is eligible for a reduced
rate of tax on dividends equal to 5% of the dividend, pro-
vided that such holder (i)is a company, (ii)qualifies for
benefits under the Treaty, (iii)holds directly at least 10%
of our voting stock, and (iv)does not conduct business
through a permanent establishment or fixed place of
business in Switzerland to which the shares or ADRs are
attributable. Such an eligible holder must apply for a
refund of the amount of the Withholding Tax in excess
of the 5% Treaty rate. Claims for refunds must be filed
on Swiss Tax Form82 (82C for corporations; 82I for indi-
viduals; 82E for other entities), which may be obtained
from any Swiss Consulate General in the US or from the
Federal Tax Administration of Switzerland at the address
below, together with an instruction form. Four copies of
the form must be duly completed, signed before a notary
public of the US, and sent to the Federal Tax Adminis-
tration of Switzerland, Eigerstrasse 65, CH-3003 Bern,
Switzerland. The form must be accompanied by suitable
evidence of deduction of Swiss tax withheld at source,
such as certificates of deduction, signed bank vouchers
or credit slips. The form may be filed on or after July1 or
January1 following the date the dividend was payable,
but no later than December31 of the third year following
the calendar year in which the dividend became payable.
For US resident holders of ADRs, JPMorgan Chase Bank,
N.A., as depositary, will comply with these Swiss

Item10. Additional Information
170
procedures on behalf of the holders, and will remit the
net amount to the holders.
Stamp Duty upon transfer of securities. The sale of
shares, whether by Swiss residents or Non-Resident
Holders, may be subject to federal securities transfer
Stamp Duty of 0.15%, calculated on the sale proceeds,
if the sale occurs through or with a Swiss bank or other
Swiss securities dealer, as defined in the Swiss Federal
Stamp Duty Act. The Stamp Duty has to be paid by the
securities dealer and may be charged to the parties in a
taxable transaction who are not securities dealers.
Stamp Duty may also be due if a sale of shares occurs
with or through a non-Swiss bank or securities dealer,
provided that (i)such bank or dealer is a member of the
SIX, and (ii)the sale takes place on the SIX. In addition
to this Stamp Duty, the sale of shares by or through a
member of the SIX may be subject to a minor stock
exchange levy.
US federal income taxation
The following is a general discussion of the material US
federal income tax consequences of the ownership and
disposition of our shares or ADRs that may be relevant
to you if you are a US Holder (as defined below). Because
this discussion does not consider any specific circum-
stances of any particular holder of our shares or ADRs,
persons who are subject to US taxation are strongly
urged to consult their own tax advisors as to the overall
US federal, state and local tax consequences, as well as
to the overall Swiss and other foreign tax consequences,
of the ownership and disposition of our shares or ADRs.
In particular, additional or dierent rules may apply to US
expatriates; banks and other financial institutions; regu-
lated investment companies; traders in securities who
elect to apply a mark-to-market method of accounting;
dealers in securities or currencies; tax-exempt entities;
insurance companies; broker-dealers; investors liable for
alternative minimum tax; investors that hold shares or
ADRs as part of a straddle, hedging or conversion trans-
action; holders whose functional currency is not the US
dollar; partnerships or other pass-through entities; per-
sons who acquired our shares pursuant to the exercise
of employee stock options or otherwise as compensa-
tion; and persons who hold, directly, indirectly or by attri-
bution, 10% or more of our outstanding shares. This dis-
cussion generally applies only to US Holders who hold
the shares or ADRs as a capital asset (generally, for
investment purposes), and whose functional currency is
the US dollar. Investors are urged to consult their own
tax advisors concerning whether they are eligible for
benefits under the Treaty.
For purposes of this discussion, a US Holder is a ben-
eficial owner of our shares or ADRs who is (i)an individ-
ual who is a citizen or resident of the US for US federal
income tax purposes; (ii)a corporation (or other entity
taxable as a corporation for US federal income tax pur-
poses) created or organized in or under the laws of the
US or a state thereof or the District of Columbia; (iii)an
estate the income of which is subject to US federal
income taxation regardless of its source; or (iv)a trust
(i)subject to the primary supervision of a US court and
the control of one or more US persons, or (ii)that has a
valid election in place to be treated as a US person. If a
partnership (or other entity treated as a partnership for
US federal income tax purposes) holds shares or ADRs,
the tax treatment of a partner generally will depend upon
the status of the partner and the activities of the part-
nership. Partners in a partnership that holds shares or
ADRs are urged to consult their own tax advisor regard-
ing the specific tax consequences of the owning and
disposing of such shares or ADRs by the partnership.
For US federal income tax purposes, a US Holder of
ADRs generally will be treated as the beneficial owner
of our shares represented by the ADRs. However, see
the discussion below under “—Dividends” regarding cer-
tain statements made by the US Treasury concerning
depositary arrangements.
This discussion assumes that each obligation in the
Deposit Agreement and any related agreement will be
performed in accordance with its terms.
Dividends. US Holders will be required to include in gross
income, as an item of ordinary income, the full amount
(without reduction for any Withholding Tax) of the divi-
dend paid with respect to our shares or ADRs at the time
that such dividend is received by the US Holder, in the
case of shares, or by the depositary, in the case of ADRs.
For this purpose, a “dividend” will include any distribu-
tion paid by us with respect to our shares or ADRs (other
than certain pro rata distributions of our capital stock)
paid out of our current or accumulated earnings and prof-
its, as determined under US federal income tax princi-
ples. To the extent the amount of a distribution by us
exceeds our current and accumulated earnings and prof-
its, such excess will first be treated as a tax-free return
of capital to the extent of a US Holder’s tax basis in the
shares or ADRs (with a corresponding reduction in such
tax basis), and thereafter will be treated as capital gain,
which will be long-term capital gain if the US Holder held
our shares or ADRs for more than one year. Under the
Code, dividend payments by us on the shares or ADRs
are not eligible for the dividends received deduction gen-
erally allowed to corporate shareholders.
Dividend income in respect of our shares or ADRs
will constitute income from sources outside the US for
US foreign tax credit purposes. Subject to the limitations
and conditions provided in the Code, US Holders gener-
ally may claim as a credit against their US federal income
tax liability, any Withholding Tax withheld from a dividend.
The rules governing the foreign tax credit are complex.
Each US Holder is urged to consult its own tax advisor
concerning whether, and to what extent, a foreign tax
credit will be available with respect to dividends received
from us. Alternatively, a US Holder may claim the With-
holding Tax as a deduction for the taxable year within
which the Withholding Tax is paid or accrued, provided
a deduction is claimed for all of the foreign income taxes
the US Holder pays or accrues in the particular year. A
deduction does not reduce US tax on a dollar-for-dollar
basis like a tax credit. The deduction, however, is not
subject to the limitations applicable to foreign tax cred-
its, but may be subject to other limitations, and each US
Holder is urged to consult its own tax advisor.
The US Treasury has expressed concern that parties
to whom ADRs are released may be taking actions

Item10. Additional Information
171
inconsistent with the claiming of foreign tax credits for
US Holders of ADRs. Accordingly, the summary above
of the creditability of the Withholding Tax could be
aected by future actions that may be taken by the US
Treasury.
In general, a US Holder will be required to determine
the amount of any dividend paid in Swiss francs, includ-
ing the amount of any Withholding Tax imposed thereon,
by translating the Swiss francs into US dollars at the spot
rate on the date the dividend is actually or constructively
received by a US Holder, in the case of shares, or by the
depositary, in the case of ADRs, regardless of whether
the Swiss francs are in fact converted into US dollars. If
a US Holder converts the Swiss francs so received into
US dollars on the date of receipt, the US Holder gener-
ally should not recognize foreign currency gain or loss
on such conversion. If a US Holder does not convert the
Swiss francs so received into US dollars on the date of
receipt, the US Holder will have a tax basis in the Swiss
francs equal to the US dollar value on such date. Any for-
eign currency gain or loss that a US Holder recognizes
on a subsequent conversion or other disposition of the
Swiss francs generally will be treated as US source ordi-
nary income or loss.
For a non-corporate US Holder, the US dollar amount
of any dividends paid that constitute qualified dividend
income generally will be taxable at a maximum rate of
15% (or 20% in the case of taxpayers with annual income
that exceeds certain thresholds), provided that the US
Holder meets certain holding period and other require-
ments. In addition, the dividends could be subject to a
3.8% net investment income tax. This tax is applied
against the lesser of the US Holder’s net investment
income or the amount by which modified adjusted gross
income exceeds a statutory threshold amount based on
filing status. We currently believe that dividends paid with
respect to our shares and ADRs will constitute qualified
dividend income for US federal income tax purposes,
provided that the US Holder meets certain holding period
and other requirements. US Holders of shares or ADRs
are urged to consult their own tax advisors regarding the
availability to them of the reduced dividend rate in light
of their own particular situation and the computations of
their foreign tax credit limitation with respect to any qual-
ified dividends paid to them, as applicable.
Sale or other taxable disposition. Upon a sale or other
taxable disposition of shares or ADRs, US Holders
generally will recognize capital gain or loss in an amount
equal to the dierence between the US dollar value of
the amount realized on the disposition and the US Hold-
er’s tax basis (determined in US dollars) in the shares or
ADRs. This capital gain or loss generally will be US
source gain or loss and will be treated as long-term cap-
ital gain or loss if the holding period in the shares or ADRs
exceeds one year. In the case of a non-corporate US
Holder, any long-term capital gain generally will be sub-
ject to US federal income tax at preferential rates, with
a maximum rate of 15% (or 20% in the case of taxpayers
with annual income that exceeds certain thresholds). In
addition, the gains could be subject to a 3.8% investment
income tax. This tax is applied against the lesser of the
US Holder’s net investment income or the amount by
which modified adjusted gross income exceeds a stat-
utory threshold amount based on filing status. The
deductibility of capital losses is subject to significant lim-
itations under the Code. Deposits or withdrawals of our
shares by US Holders in exchanges for ADRs will not
result in the realization of gain or loss for US federal
income tax purposes.
US information reporting and backup withholding. Divi-
dend payments with respect to shares or ADRs and pro-
ceeds from the sale, exchange or other disposition of
shares or ADRs received in the United States or through
US-related financial intermediaries may be subject to
information reporting to the US Internal Revenue Service
(IRS) and possible US backup withholding. Certain
exempt recipients (such as corporations) are not subject
to these information reporting and backup withholding
requirements. Backup withholding will not apply to a US
Holder who furnishes a correct taxpayer identification
number and makes any other required certification or
who is otherwise exempt from backup withholding. Any
US Holders required to establish their exempt status
generally must provide a properly executed IRS FormW-9
(Request for Taxpayer Identification Number and Certi-
fication). Backup withholding is not an additional tax.
Amounts withheld as backup withholding may be cred-
ited against a US Holder’s US federal income tax liabil-
ity, and a US Holder may obtain a refund of any excess
amounts withheld under the backup withholding rules by
timely filing the appropriate claim for refund with the IRS
and furnishing any required information.
10.F Dividends and paying agents
Not applicable.
10.G Statement by experts
Not applicable.

Item10. Additional Information
172
10.H Documents on display
Any statement in this Form20-F about any of our con-
tracts or other documents is not necessarily complete.
If the contract or document is filed as an exhibit to the
Form20-F, the contract or document is deemed to mod-
ify the description contained in this Form20-F. You must
review the exhibits themselves for a complete descrip-
tion of the contract or document.
The SEC maintains an internet site at http://www.sec.
gov that contains reports and other information regard-
ing issuers that file electronically with the SEC. These
SEC filings are also available to the public from commer-
cial document retrieval services.
We are required to file or furnish reports and other
information with the SEC under the Exchange Act and
regulations under that act. As a foreign private issuer, we
are exempt from the rules under the Exchange Act pre-
scribing the form and content of proxy statements, and
our ocers, directors and principal shareholders are
exempt from the reporting and short-swing profit recov-
ery provisions contained in Section16 of the Exchange
Act.
10.I Subsidiary information
Not applicable.

Item11. Quantitative and Qualitative Disclosures About Market Risk
173
Item11. Quantitative and Qualitative
Disclosures About Market Risk
The major financial risks facing the Group are managed
centrally by Group Treasury, which has established pro-
cesses and procedures to identify, aggregate and man-
age our financial risk exposure. The Group Treasury
function is included in management’s internal control
assessment.
For information about the eects of currency fluctu-
ations and how we manage currency risk, see “Item5.
Operating and Financial Review and Prospects—Item5.B
Liquidity and capital resources.”
The information set forth under “Item 18. Financial
Statements—Note29. Financial instruments—additional
disclosures” is incorporated by reference.

Item12. Description of Securities Other Than Equity Securities
174
Item12. Description of Securities Other Than
Equity Securities
12.A Debt securities
Not applicable.
12.B Warrants and rights
Not applicable.
12.C Other securities
Not applicable.
12.D American Depositary Shares
Fees payable by ADR holders
According to our Deposit Agreement with the ADS depositary, JPMorgan Chase Bank, N.A. (JPMorgan), holders
of our ADRs may have to pay to JPMorgan, either directly or indirectly, fees or charges up to the amounts set forth
below:
Category Depositary actions Associated fee
Depositing or substituting Acceptance of shares surrendered, and issuance of ADRs in exchange, USD for each ADSs
underlying shares including surrenders and issuances in respect of: (or portion thereof)
— Share distributions evidenced by the new
— Stock split ADRs delivered
— Rights
— Merger
— Exchange of shares or any other transaction or event or other distribution
aecting the ADSs or the deposited shares
Withdrawing Acceptance of ADRs surrendered for withdrawal of deposited shares USD for each ADSs
underlying shares (or portion thereof)
evidenced by the ADRs
surrendered
Selling or Distribution or sale of shares, the fee being in an amount equal to the fee USD for each ADSs
exercising rights for the execution and delivery of ADRs that would have been charged (or portion thereof)
as a result of the deposit of such shares
Transferring, Transfers, combining or grouping of depositary receipts USD per ADR
splitting or
grouping receipts
Expenses of the Expenses incurred on behalf of holders in connection with: Expenses payable at the sole
depositary — Compliance with foreign exchange control regulations or any law or discretion of the depositary
regulation relating to foreign investment by billing holders or by
— The depositary’s or its custodian’s compliance with applicable law, deducting charges from one
rule or regulation or more cash dividends or
— Stock transfer or other taxes and other governmental charges other cash distributions
— Cable, telex and facsimile transmission and delivery
— Expenses of the depositary in connection with the conversion of foreign
currency into US dollars (which are paid out of such foreign currency)
— Any other charge payable by any of the depositary or its agents
Advance tax relief Tax relief/reclamation process for qualified holders A depositary service charge
of USD per ADS

Item12. Description of Securities Other Than Equity Securities
175
Fees payable by the depositary to the
issuer
Pursuant to an agreement eective as of May11, 2017
(“the Agreement”), JPMorgan, as our ADS depositary,
has agreed to make an annual contribution payment to
Novartis at the end of each 12-month period beginning
on the eective date of the Agreement and on each sub-
sequent anniversary of the eective date of the Agree-
ment (each such 12-month period is a “Contract Year”).
This annual contribution payment will equal: (a)(1) USD
1.7million less (a)(2) the custody costs, fees and expenses
(including, without limitation, any central securities
depository fees, charges and expenses) incurred during
the applicable Contract Year (the items in (a)(2) collec-
tively are the “Custody Costs”) plus (b)70% of the gross
issuance and cancellation fees collected by JPMorgan
under the Deposit Agreement during such Contract Year
minus (c)that portion (if any) of JPMorgan’s legal fees,
charges and out-of-pocket expenses in excess of USD
50 000 for such Contract Year. To the extent that the
Custody Costs for a Contract Year exceed USD 1.7mil-
lion, these costs would be capped at USD 1.7million.
JPMorgan has further agreed to waive the USD 0.05
per ADS issuance fees that would normally be owed by
Novartis in connection with our deposits of shares as
part of our employee stock ownership and employee par-
ticipation plans. Novartis is responsible for reimbursing
JPMorgan for all taxes and governmental charges
required to have been withheld and/or paid, and not so
withheld and/or paid, arising from such waived fees.

Item13. Defaults, Dividend Arrearages and Delinquencies
176
PART II
Item13. Defaults, Dividend Arrearages and
Delinquencies
None.

Item14. Material Modifications to the Rights of Security Holders and Use of Proceeds
177
Item14. Material Modifications to the Rights
of Security Holders and Use of Proceeds
None.

Item15. Controls and Procedures
178
Item15. Controls and Procedures
Report of Novartis Management on Internal Control Over Financial Reporting
Novartis AG’s Chief Executive Ocer and Chief Finan‑
cial Ocer, after evaluating the eectiveness of our dis‑
closure controls and procedures (as defined in Exchange
Act Rule13a‑15(e)) as of the end of the period covered
by this Annual Report, have concluded that, as of such
date, our disclosure controls and procedures were eec‑
tive.
The Board of Directors and management of the
Group are responsible for establishing and maintaining
adequate internal control over financial reporting. The
Group’s internal control system was designed to provide
reasonable assurance to the Group’s management and
Board of Directors regarding the reliability of financial
reporting and the preparation and fair presentation of its
published consolidated financial statements.
All internal control systems, no matter how well
designed, have inherent limitations. Therefore, even
those systems determined to be eective may not pre‑
vent or detect misstatements and can provide only rea‑
sonable assurance with respect to financial statement
preparation and presentation. Also, projections of any
evaluation of eectiveness to future periods are subject
to the risk that controls may become inadequate because
of changes in conditions, or that the degree of compli‑
ance with the policies or procedures may deteriorate.
Group management assessed the eectiveness of
the Group’s internal control over financial reporting as
of December31, 2022. In making this assessment, it used
the criteria established in Internal Control—Integrated
Framework (2013) issued by the Committee of Sponsor‑
ing Organizations of the Treadway Commission (COSO).
Based on our assessment, management concluded that,
as of December31, 2022, the Group’s internal control
over financial reporting is eective based on those cri‑
teria.
KPMG AG, Switzerland, an independent registered
public accounting firm, has issued an unqualified opin‑
ion on the eectiveness of the Group’s internal control
over financial reporting, which is included in this Annual
Report under “Item18. Financial Statements—Report of
independent registered public accounting firm.”
See the report of KPMG, an independent registered
public accounting firm, included under “Item18. Finan‑
cial Statements—Report of independent registered pub‑
lic accounting firm.”
There were no changes to our internal control over
financial reporting that occurred during the period cov‑
ered by this Annual Report that have materially aected,
or are reasonably likely to materially aect, our internal
control over financial reporting.
Vas Narasimhan Harry Kirsch
Chief Executive Ocer Chief Financial Ocer
Basel, January 31, 2023

Item16A. Audit Committee Financial Expert
179
Item16A. Audit Committee Financial Expert
Our Audit and Compliance Committee has determined
that Elizabeth Doherty and Ana de Pro Gonzalo possess
specific accounting and financial management exper-
tise, and that they are Audit Committee Financial Experts
as defined by the SEC. The Board of Directors has also
determined that Elizabeth Doherty and Ana de Pro
Gonzalo are “independent” in accordance with the appli
-
cable requirements of Rule10A-3 of the Exchange Act,
and that other members of the Audit and Compliance
Committee have sucient experience and ability in
finance and compliance matters to enable them to ade-
quately discharge their responsibilities.

Item16B. Code of Ethics
180
Item16B. Code of Ethics
In addition to our Code of Ethics and Professional Prac-
tices Policy, which are applicable to all of our employees,
we have adopted Ethical Conduct Requirements that
impose additional obligations on our principal executive
ocer, principal financial ocer, principal accounting
ocer, and persons performing similar functions. This
document is accessible on our internet website at:
https://www.novartis.com/investors/company-over-
view/corporate-governance

Item16C. Principal Accountant Fees and Services
181
Item16C. Principal Accountant Fees and
Services
The information set forth under “Item 6. Directors, Senior Management and Employees—Item 6.C Board practices—
Corporate governance—Auditors” is incorporated by reference.

Item16D. Exemptions from the Listing Standards for Audit Committees
182
Item16D. Exemptions from the Listing
Standards for Audit Committees
Not applicable.

Item16E. Purchases of Equity Securities by the Issuer and Aliated Purchasers
183
Item16E. Purchases of Equity Securities by
the Issuer and Aliated Purchasers
Maximum
Maximum
approximate
approximate
Total number
value of
value of
of shares
shares that
shares that
purchased
may yet be
may yet be
as part of
purchased
purchased
publicly
under the
under the
Average price
announced
plans or
plans or
Total number of
paid per share
plans or
programs
programs
shares purchased
in USD
programs
(CHF millions)
(USD millions)
(a)
(b)
(c)
(d)
(e)
Jan. -
Feb. -
Mar. -
Apr. -
May -
Jun. -
Jul. -
Aug. -
Sep. -
Oct. -
Nov. -
Dec. -
Total
1
Column (a) shows shares repurchased on the SIX Swiss Exchange second trading line plus shares we purchased from employees who had
obtained the shares through a Novartis Employee Ownership Plan. See “Item 18. Financial Statements – Note 26 Equity-based participation
plans for employees.”
2
Column (c) shows shares repurchased on the SIX Swiss Exchange second trading line under the CHF 10 billion share buyback authority
approved at the 2021 AGM and under the additional CHF 10 billion share buyback authority approved at the 2022 AGM for transactions in
2022. See “Item 6. Directors, Senior Management and Employees – Item 6C. Board Practices – Our capital structure – Changes in capital.”
3
Column (e) shows the Swiss franc amount from column (d) converted into US dollars as of the month-end, using the Swiss franc/US dollar
exchange rate at the applicable month-end

Item16F. Change in Registrant’s Certifying Accountant
184
Item16F. Change in Registrant’s Certifying
Accountant
Not applicable.

Item16G. Corporate Governance
185
Item16G. Corporate Governance
Novartis AG is subject to and compliant with the laws
and regulations of Switzerland (in particular, Swiss com-
pany and securities laws, SIX Swiss Exchange rules and
the Swiss Code of Best Practice for Corporate Gover-
nance) and the securities laws of the United States,
including New York Stock Exchange (NYSE) rules, as
applicable to foreign private issuers of securities. The
following summarizes some significant ways in which our
corporate governance practices dier from those fol-
lowed by domestic listed US companies under the list-
ing standards of the NYSE:
• Novartis AG shareholders do not receive written
reports directly from Board committees.
• External auditors are appointed by shareholders at the
Annual General Meeting of Shareholders (AGM), as
opposed to being appointed by the Audit and Compli-
ance Committee.
• While shareholders cannot vote on all equity compen-
sation plans, they are entitled to hold separate, yearly
binding votes on Board and Executive Committee com-
pensation.
• The Board has set up a separate Risk Committee that
oversees the risk management system and processes,
as opposed to delegating this responsibility to the Audit
and Compliance Committee.
• The full Board is responsible for overseeing the
performance evaluation of the Board and Executive
Committee.
• The full Board is responsible for setting objectives rel-
evant to the CEO’s compensation and for evaluating
his performance.

Item16H. Mine Safety Disclosure
186
Item16H. Mine Safety Disclosure
Not applicable.

Item16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
187
Item16I. Disclosure Regarding Foreign
Jurisdictions that Prevent Inspections
Not applicable.

Item17. Financial Statements
188
PART III
Item17. Financial Statements
See response to “Item18. Financial Statements.”

Item18. Financial Statements
189
Item18. Financial Statements
The following financial statements are filed as part of this Annual Report.
Page
Consolidated income statements F-
Consolidated statements of comprehensive income F-
Consolidated balance sheets F-
Consolidated statements of changes in equity F-
Consolidated statements of cash flows F-
Notes to the Novartis Group consolidated financial statements F-
. Significant accounting policies F-
. Significant transactions F-
. Segmentation of key figures , and F-
. Associated companies F-
. Interest expense andotherfinancialincomeandexpense F-
. Income taxes F-
. Earnings per share F-
. Changes in consolidated statements ofcomprehensive income F-
. Property, plant and equipment F-
. Right-of-use assets and lease liabilities F-
. Goodwill and intangible assets F-
. Deferred tax assets and liabilities F-
. Financial and other non-current assets F-
. Inventories F-
. Trade receivables F-
. Marketable securities, commodities, timedeposits, derivative financial instruments,
andcash and cash equivalents F-
. Other current assets F-
. Equity F-
. Non-current financial debt F-
. Provisions and other non-current liabilities F-
. Current financial debt andderivativefinancialinstruments F-
. Provisions and other current liabilities F-
. Details to the consolidated statements of cash flows F-
. Acquisitions of businesses F-
. Post-employment benefits for employees F-
. Equity-based participation plans for employees F-
. Transactions with related parties F-
. Commitments and contingent liabilities F-
. Financial instruments – additional disclosures F-
. Events subsequent to the December , , consolidated balance sheet date F-
. Principal Group subsidiaries andassociatedcompanies F-
Statutory Auditor’s Report on the consolidated financial statements of Novartis AG F-
Financial statements of Novartis AG A-
Notes to the financial statements of Novartis AG A-
Appropriation of available earnings and reserves of Novartis AG A-
Statutory Auditor’s Report on the financial statements of Novartis AG A-

Item19. Exhibits
190
Item19. Exhibits
The SEC maintains an internet site at http://www.sec.gov that contains reports and other information regarding
issuers that file electronically with the SEC. These SEC filings are also available to the public from commercial doc-
ument retrieval services.
1.1 Articles of Incorporation of Novartis AG, as amended March 2, 2021 (English translation) (incorporated
by reference to Exhibit4.1 to Novartis AG’s registration statement on FormS-8 (File No.333-258081) as
filed with the SEC on July 22, 2021).
1.2 Regulations of the Board of Directors, the Board Committees and the Executive Committee of Novartis
AG, eective January 1, 2021 (incorporated by reference to Exhibit 1.2 to Novartis AG’s Annual Report
on Form 20-F (File No. 001-15024) as filed with the SEC on January 26, 2021).
2.1 Form of Second Amended and Restated Deposit Agreement among Novartis AG, JPMorgan Chase Bank,
N.A., as depositary, and all Holders and Beneficial Owners from time to time of American Depositary
Receipts issued thereunder (incorporated by reference to Exhibit99.A to the Registration Statement on
FormF-6 (File No.333-198623) as filed with the SEC on December 16, 2022).
2.2 Form of American Depositary Receipt (incorporated by reference to Exhibit99.A to the Registration
Statement on FormF-6 (File No.333-198623) as filed with the SEC on December 16, 2022).
2.3 The total amount of long-term debt securities authorized under any instrument does not exceed 10% of
the total assets of the Company and its subsidiaries on a consolidated basis. We hereby agree to furnish
to the SEC, upon its request, a copy of any instrument defining the rights of holders of long-term debt of
the Company or of its subsidiaries for which consolidated or unconsolidated financial statements are
required to be filed.
2.4 Description of Securities registered under Section 12 of the Exchange Act.
8.1 For a list of all of our principal Group subsidiaries and associated companies, see “Item 18. Financial
Statements—Note31. Principal Group subsidiaries and associated companies.”
12.1 Certification of Vasant Narasimhan, Chief Executive Ocer of Novartis AG, pursuant to Section302 of
the Sarbanes-Oxley Act of 2002.
12.2 Certification of Harry Kirsch, Chief Financial Ocer of Novartis AG, pursuant to Section302 of the Sar-
banes-Oxley Act of 2002.
13.1 Certification of Vasant Narasimhan, Chief Executive Ocer of Novartis AG, pursuant to Section18 U.S.C.
Section1350, as adopted pursuant to Section906 of the Sarbanes-Oxley Act of 2002.
13.2 Certification of Harry Kirsch, Chief Financial Ocer of Novartis AG, pursuant to Section18 U.S.C. Sec-
tion1350, as adopted pursuant to Section906 of the Sarbanes-Oxley Act of 2002.
15.1 Consent of KPMG AG.
15.2 Consent of PricewaterhouseCoopers AG.

Item19. Exhibits
191
101.INS XBRL Instance Document
101.SCH XBRL Taxonomy Extension Schema Document
101.CAL XBRL Taxonomy Extension Calculation Linkbase Document
101.DEF XBRL Taxonomy Extension Definition Linkbase Document
101.LAB XBRL Taxonomy Extension Label Linkbase Document
101.PRE XBRL Taxonomy Extension Presentation Linkbase Document
104 Cover Page Interactive Data File (embedded within the Inline XBRL document and included in Exhibit101).

192
(This page has been left blank intentionally.)

Novartis Group consolidatedfinancialstatements
F-1
Novartis Group
consolidatedfinancialstatements
Consolidated income statements
(For the years ended December 31, 2022, 2021 and 2020)
(USD millions unless indicated otherwise) Note
Net sales to third parties
Other revenues
Cost of goods sold
–
–
–
Gross profit
Selling, general and administration
–
–
–
Research and development
–
–
–
Other income
Other expense
–
–
–
Operating income
(Loss)/income from associated companies
–
Interest expense
–
–
–
Other financial income and expense
–
–
Income before taxes
Income taxes
–
–
–
Net income
Attributable to:
Shareholders of Novartis AG
6 955
24 021
8 072
Non-controlling interests
0
– 3
– 1
Basic earnings per share (USD)
Diluted earnings per share (USD)
The accompanying Notes form an integral part of the consolidated financial statements.

Novartis Group consolidatedfinancialstatements
F-2
Consolidated statements of comprehensive income
(For the years ended December 31, 2022, 2021 and 2020)
(USD millions) Note
Net income
Other comprehensive income
Items that are or may be recycled into the consolidated income statement
Novartis share of other comprehensive income
recognized by associated companies, net of taxes
–
Net investment hedge, net of taxes
–
Currency translation eects, net of taxes
–
–
Total of items that are or may be recycled
–
–
Items that will never be recycled into the consolidated income statement
Actuarial (losses)/gains from defined benefit plans, net of taxes
–
Fair value adjustments on equity securities, net of taxes
–
Total of items that will never be recycled
–
Total comprehensive income
Attributable to:
Shareholders of Novartis AG
6 116
21 528
11 403
Non-controlling interests
– 5
– 7
– 2
The accompanying Notes form an integral part of the consolidated financial statements.

Novartis Group consolidatedfinancialstatements
F-3
Consolidated balance sheets
(At December 31, 2022 and 2021)
(USD millions) Note
Assets
Non-current assets
Property, plant and equipment
Right-of-use assets
Goodwill
Intangible assets other than goodwill
Investments in associated companies
Deferred tax assets
Financial assets
Other non-current assets
Total non-current assets
Current assets
Inventories
Trade receivables
Income tax receivables
Marketable securities, commodities, time deposits and derivative financial instruments
Cash and cash equivalents
Other current assets
Total current assets
Total assets
Equity and liabilities
Equity
Share capital
Treasury shares
–
–
Reserves
Equity attributable to Novartis AG shareholders
Non-controlling interests
Total equity
Liabilities
Non-current liabilities
Financial debts
Lease liabilities
Deferred tax liabilities
Provisions and other non-current liabilities
Total non-current liabilities
Current liabilities
Trade payables
Financial debts and derivative financial instruments
Lease liabilities
Current income tax liabilities
Provisions and other current liabilities
Total current liabilities
Total liabilities
Total equity and liabilities
The accompanying Notes form an integral part of the consolidated financial statements.

Novartis Group consolidatedfinancialstatements
F-4
Consolidated statements of changes in equity
(For the years ended December 31, 2022, 2021 and 2020)
Reserves
Equity
attributable
Non-
Share
Treasury
Retained
Total value
to Novartis
controlling
Total
(USD millions) Note
capital
shares
earnings
adjustments
shareholders
interests
equity
Total equity at January ,
–
–
Net income
–
Other comprehensive income
–
–
Total comprehensive income
–
Dividends
–
–
–
Purchase of treasury shares
–
–
–
–
Reduction of share capital
–
–
Exercise of options and employee transactions
Repurchase of options
–
–
–
Equity-based compensation
Shares delivered to Alcon employees
as a result of the Alcon spin-o
Taxes on treasury share transactions
Increase of treasury share repurchase
obligation under a share buyback trading plan
–
–
–
Fair value adjustments on financial assets sold
–
Value adjustments related to divestments
–
Impact of change in ownership of consolidated entities
–
–
–
Other movements
Total of other equity movements
–
–
–
–
–
–
Total equity at December ,
– –
Net income
–
Other comprehensive income
–
–
–
–
Total comprehensive income
–
–
Dividends
–
–
–
Purchase of treasury shares
–
–
–
–
Reduction of share capital
–
–
Exercise of options and employee transactions
Equity-based compensation
Shares delivered to Alcon employees
as a result of the Alcon spin-o
Taxes on treasury share transactions
Increase of treasury share repurchase
obligation under a share buyback trading plan
–
–
–
Transaction costs, net of taxes
Changes in non-controlling interests
–
–
Fair value adjustments on financial assets sold
–
Value adjustments related to divestments
–
Impact of change in ownership of consolidated entities
–
–
Other movements
Total of other equity movements
–
–
–
–
–
Total equity at December ,
– –
Net income
Other comprehensive income
–
–
–
–
Total comprehensive income
–
–
Dividends
–
–
–
Purchase of treasury shares
–
–
–
–
Reduction of share capital
–
–
Exercise of options and employee transactions
Equity-based compensation
Shares delivered to Alcon employees
as a result of the Alcon spin-o
Taxes on treasury share transactions
Decrease of treasury share repurchase
obligation under a share buyback trading plan
Changes in non-controlling interests
–
–
Fair value adjustments on financial assets sold
–
Value adjustments related to divestments
–
Other movements
Total of other equity movements
–
–
–
–
–
–
Total equity at December ,
–
–
The accompanying Notes form an integral part of the consolidated financial statements.
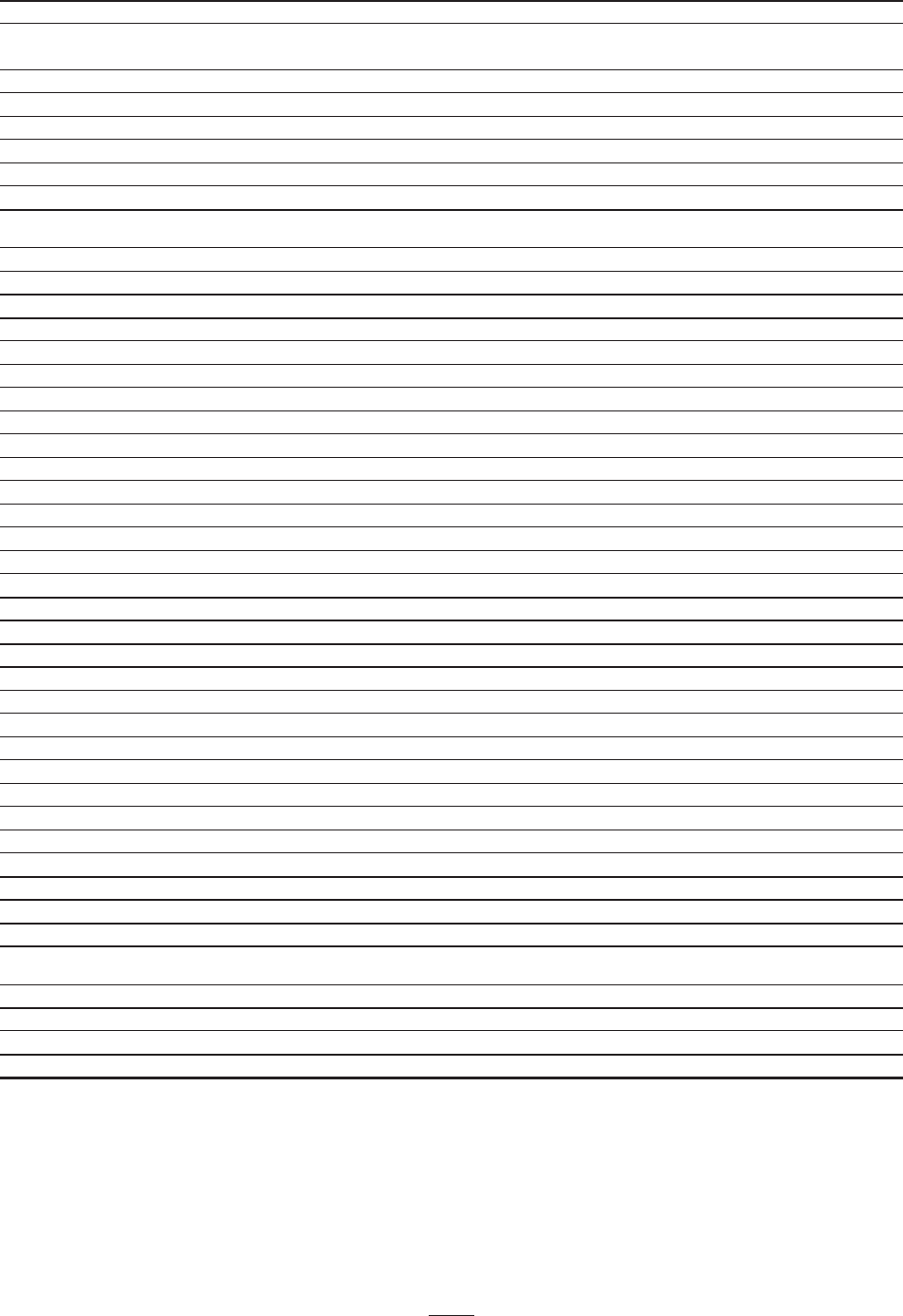
Novartis Group consolidatedfinancialstatements
F-5
Consolidated statements of cash flows
(For the years ended December 31, 2022, 2021 and 2020)
(USD millions) Note
Net income
Adjustments to reconcile net income to net cash flows from operating activities
Reversal of non-cash items and other adjustments
–
Dividends received from associated companies and others
Interest received
Interest paid
–
–
–
Other financial receipts
Other financial payments
–
–
–
Income taxes paid
–
–
–
Net cash flows from operating activities before working capital and
provision changes
Payments out of provisions and other net cash movements in non-current liabilities
–
–
–
Change in net current assets and other operating cash flow items
–
–
Net cash flows from operating activities
Purchases of property, plant and equipment
–
–
–
Proceeds from sale of property, plant and equipment
Purchases of intangible assets
–
–
–
Proceeds from sale of intangible assets
Purchases of financial assets
–
–
–
Proceeds from sale of financial assets
Purchases of other non-current assets
–
–
–
Proceeds from sale of other non-current assets
Acquisitions and divestments of interests in associated companies, net
–
–
Acquisitions and divestments of businesses, net
–
–
–
Purchases of marketable securities, commodities and time deposits
–
–
–
Proceeds from sale of marketable securities, commodities and time deposits
Net cash flows from/(used in) investing activities from continuing operations
–
Net cash flows used in investing activities from discontinued operations
–
Net cash flows from/(used in) investing activities
–
Dividends paid to shareholders of Novartis AG
–
–
–
Acquisitions of treasury shares
–
–
–
Proceeds from exercised options and other treasury share transactions, net
Increase in non-current financial debts
Repayments of the current portion of non-current financial debts
–
–
–
Change in current financial debts
–
Payments of lease liabilities
–
–
–
Impact of change in ownership of consolidated entities
–
–
Other financing cash flows, net
–
Net cash flows used in financing activities from continuing operations
–
–
–
Net cash flows used in financing activities from discontinued operations
–
Net cash flows used in financing activities
–
–
–
Net change in cash and cash equivalents before eect of exchange
rate changes
–
–
Eect of exchange rate changes on cash and cash equivalents
–
–
Net change in cash and cash equivalents
–
–
Cash and cash equivalents at January
Cash and cash equivalents at December
The accompanying Notes form an integral part of the consolidated financial statements.

Notes to the Novartis Groupconsolidatedfinancial statements
F-6
Notes to the Novartis
Groupconsolidatedfinancial statements
1. Significant accounting policies
The Novartis Group (Novartis or Group) is a multinational
group of companies specializing in the research, devel-
opment, manufacturing and marketing of a broad range
of innovative pharmaceuticals and cost-saving generic
medicines. The Group is headquartered in Basel, Swit-
zerland.
The consolidated financial statements of the Group
are prepared in accordance with International Financial
Reporting Standards (IFRS) as issued by the Interna-
tional Accounting Standards Board (IASB). They are pre-
pared in accordance with the historical cost convention,
except for items that are required to be accounted for
at fair value.
The Group’s financial year-end is December 31, which
is also the annual closing date of the individual entities’
financial statements incorporated into the Group’s con-
solidated financial statements.
The preparation of financial statements requires
management to make certain estimates and assump
-
tions, either at the balance sheet date or during the year,
which aect the reported amounts of revenues, expenses,
assets, liabilities and contingent amounts.
Estimates are based on historical experience and
other assumptions that are considered reasonable under
the given circumstances and are regularly monitored.
Actual outcomes and results could dier from those esti-
mates and assumptions. Revisions to estimates are rec-
ognized in the period in which the estimate is revised.
Listed below are accounting policies of significance to
Novartis or, in cases where IFRS provides alternatives,
the option adopted by Novartis.
Scope of consolidation
The consolidated financial statements include all enti-
ties, including structured entities, over which Novartis
AG, Basel, Switzerland, directly or indirectly has control
(generally as a result of owning more than 50% of the
entity’s voting interest). Consolidated entities are also
referred to as “subsidiaries.”
In cases where Novartis does not fully own a subsid-
iary, it has elected to value any remaining outstanding
non-controlling interest at the time of acquiring control
of the subsidiary at its proportionate share of the fair
value of the net identified assets.
Investments in associated companies (generally
defined as investments in entities in which Novartis holds
between 20% and 50% of voting shares or over which it
otherwise has significant influence) and joint ventures
are accounted for using the equity method, except for
selected venture fund investments for which the Group
has elected to apply the method of fair value through the
consolidated income statement.
Foreign currencies
The consolidated financial statements of Novartis are
presented in US dollars (USD). The functional currency
of a subsidiary is generally the local currency of that
respective entity. The functional currency used for the
reporting of certain Swiss and foreign finance entities is
USD instead of their respective local currencies. This
reflects the fact that the cash flows and transactions of
these entities are primarily denominated in this currency.
For subsidiaries not operating in hyperinflationary
economies, the subsidiary’s results, financial position
and cash flows that do not have USD as their functional
currency are translated into USD using the following
exchange rates:
• Income, expense and cash flows for each month using
the average exchange rate, with the US dollar values
for each month being aggregated during the year
• Balance sheet using year-end exchange rates
• Resulting exchange rate dierences are recognized in
other comprehensive income
For subsidiaries operating in hyperinflationary econo-
mies, the impact of the restatement of the non-monetary
assets and liabilities with the general price index at the
beginning of the period is recorded in retained earnings
in equity. The subsequent gains or losses resulting from
the restatement of non-monetary assets are recorded
in “Other financial income and expense” in the consoli-
dated income statement.
Non-current assets held for sale or
held for distribution to owners
Non-current assets are accounted for as assets held for
sale or as related to discontinued operations when their
carrying amount is to be recovered principally through
a sale transaction or distribution to owners and a sale or
distribution to owners is considered highly probable.
They are stated at the lower of carrying amount and fair
value less costs to sell and any resulting impairment is
recognized. Assets related to discontinued operations
and assets of a disposal group held for sale are not
depreciated or amortized. The prior year consolidated
balance sheet is not restated.
If in a subsequent period, the criteria for classifica-
tion as held for sale are no longer met, the recoverable
amount of assets and liabilities are reclassified out of
assets held for sale into the respective balance sheet
lines and the prior year consolidated balance sheet is
not restated. The cumulative amount of depreciation and
amortization not recorded since the date of their classi-
fication as assets held for sale, and any required

Notes to the Novartis Groupconsolidatedfinancial statements
F-7
adjustments to the recoverable amounts of assets are
recognized in the consolidated income statement.
Acquisition of assets and businesses
Assets separately acquired are recorded at cost, which
includes the purchase price and any directly attributable
costs for bringing the asset into the condition to operate
as intended. Expected costs for obligations to disman-
tle and remove property, plant and equipment and restore
the site when it is no longer used are included in their
cost.
Acquired businesses are accounted for by applying
the acquisition method, unless the optional concentra-
tion test is applied. The optional concentration test
allows for an election on a transaction-by-transaction
basis to account for the acquired business as an asset
separately acquired when substantially all of the fair
value of the gross assets acquired is concentrated in a
single identifiable asset or group of similar identifiable
assets.
The acquisition method requires that the assets
acquired and liabilities assumed be recorded at their
respective fair values on the date the Group obtains con-
trol. The excess of the fair value of the total purchase
consideration transferred over the fair value of the
acquired assets and assumed liabilities is recognized as
goodwill. The valuations are based on information avail-
able at the acquisition date. Acquisition related costs are
expensed as incurred.
The application of the acquisition method requires
certain estimates and assumptions to be made, espe-
cially concerning the fair values of the acquired intangi-
ble assets, inventories, property, plant and equipment
and the liabilities assumed at the acquisition date, and
the useful lives of the intangible assets and property,
plant and equipment. Estimates of fair value require the
use of valuation techniques. These valuations require the
use of management assumptions and estimates, includ-
ing the value of comparable assets in the market, amount
and timing of future cash flows, outcomes and costs of
research and development activities, probability of
obtaining regulatory approval, long-term sales forecasts,
actions of competitors, discount rates and terminal
growth rates. The section “—Impairment of goodwill and
intangible assets” in this Note 1 provides additional infor-
mation on key assumptions that are highly sensitive in
the estimation of fair values using valuation techniques.
Property, plant and equipment
Property, plant and equipment is depreciated on a
straight-line basis in the consolidated income statement
over the estimated useful life of the individual asset. Free
-
hold land is not depreciated. The related depreciation
expense is included in the costs of the functions using
the asset.
Property, plant and equipment is assessed for impair
-
ment whenever there is an indication that the balance
sheet carrying amount may not be recoverable using
cash flow projections over the useful life.
The following table shows the estimated useful life
by major categories for property, plant and equipment:
Useful life
Buildings to years
Machinery and other equipment
Machinery and equipment to years
Furniture and vehicles to years
Computer hardware to years
Government grants obtained for construction activities,
including any related equipment, are deducted from the
gross acquisition cost to arrive at the balance sheet car-
rying value of the related assets.
Leases and right-of-use assets
As lessee, at inception and upon the modification of a
contract, the Group assesses whether the contract con-
tains a lease. The Group elected to allocate the consid-
eration in the contract to the lease and non-lease com-
ponents on the basis of the relative standalone price of
each component.
The Group recognizes a right-of-use asset and a cor-
responding lease liability for all arrangements in which
it is a lessee, except for leases with a term of 12 months
or less (short-term leases) and low-value leases. For
these short-term and low-value leases, the Group rec-
ognizes the lease payments as an operating expense on
a straight-line basis over the term of the lease.
The lease liability is initially measured at the present
value of the future lease payments as from the com-
mencement date of the lease to the end of the lease term.
The lease term includes the period of any lease exten-
sion that management assess as reasonably certain to
be exercised by the Group. The lease payments are dis-
counted using the interest rate implicit in the lease or, if
not readily determinable, the Novartis incremental bor-
rowing rate for the asset subject to the lease in the rel-
evant market.
The Group remeasures the lease liability (and makes
a corresponding adjustment to the related right-of-use
asset) whenever there is a change to the lease terms or
expected payments under the lease, or a modification
that is not accounted for as a separate lease.
The portion of the lease payments attributable to the
repayment of lease liabilities is recognized in cash flows
used in financing activities, and the portion attributable
to the payment of interest is included in cash flows from
operating activities.
Right-of-use assets are initially recognized on the bal-
ance sheet at cost, which comprises the amount of the
initial measurement of the corresponding lease liability,
adjusted for any lease payments made at or prior to the
commencement date of the lease, any lease incentive
received, and any initial direct costs incurred by Novartis,
and expected costs for obligations to dismantle and
remove right-of-use assets when they are no longer
used.
Right-of-use assets are depreciated on a straight-line
basis from the commencement date of the lease over

Notes to the Novartis Groupconsolidatedfinancial statements
F-8
the shorter of the useful life of the right-of-use asset or
the end of the lease term.
Right-of-use assets are assessed for impairment
whenever there is an indication that the balance sheet
carrying amount may not be recoverable using cash flow
projections for the useful life.
In arrangements where the Group is the lessor, it
determines at lease inception whether the lease is a
finance lease or an operating lease. Leases that trans-
fer substantially all of the risk and rewards incidental to
ownership of the underlying asset to the counterparty
(the lessee) are accounted for as finance leases. Leases
that do not transfer substantially all of the risks and
rewards of ownership are accounted for as operating
leases. Operating lease payments received are recog-
nized on a straight-line basis over the lease term in the
consolidated income statement in “Other income.”
Goodwill and intangible assets
Goodwill
Goodwill arises on applying the acquisition method on
the acquisition of a business and is the excess of the fair
value of the consideration transferred to acquire the
business over the underlying fair value of the net identi-
fied assets acquired. It is allocated to groups of cash-gen-
erating units (CGUs), that are expected to benefit from
the synergies of the combination, and which are usually
represented by the reported segments. Goodwill is
tested for impairment annually at the level of these
groups of CGUs, and any impairment charges are
recorded under “Other expense” in the consolidated
income statement.
Intangible assets available for use
Novartis has the following classes of available for use
intangible assets: currently marketed products; technol-
ogies and other intangible assets (including software).
Currently marketed products represent the compos-
ite value of acquired intellectual property (IP), patents,
distribution rights and product trade names.
Technologies represent identified and separable
acquired know-how used in the research, development
and production processes.
Significant investments in internally developed and
acquired computer software are capitalized and included
in the “Other” category, and amortized once available for
use.
Intangible assets available for use with a definite use-
ful life are amortized over their estimated useful lives on
a straight-line basis and are evaluated for potential
impairment whenever facts and circumstances indicate
that their carrying value may not be recoverable.
The following table shows the estimated useful life
by major categories for intangible assets available for
use and the line in the consolidated income statement
in which the amortization and any potential impairment
charge is recognized:
Income statement line
for amortization and
Useful life
impairment charges
Currently marketed products 5 to 20 years
“Cost of goods sold”
Technologies 10 to 20 years
“Cost of goods sold”
or “Research
and development”
Other (including 3 to 12 years
In the relevant
software)
functional expense
Intangible assets not yet available for use
Acquired research and development intangible assets
that have not yet obtained marketing approval are rec-
ognized as in-process research and development
(IPR&D).
IPR&D is not amortized, but is evaluated for potential
impairment on an annual basis or when facts and circum
-
stances warrant. Any impairment charge is recorded in
the consolidated income statement under “Research and
development.” Once a project included in IPR&D has
received marketing approval from a regulatory authority,
it is transferred to the “Currently marketed products” cat-
egory.
Impairment of goodwill and intangible
assets
An asset, a CGU or a grouping of CGUs is considered
impaired when its balance sheet carrying amount
exceeds its estimated recoverable amount, which is
defined as the higher of its fair value less costs of dis-
posal and its value in use. Usually, Novartis applies the
fair value less costs of disposal method for its impair-
ment assessment. In most cases, no directly observable
market inputs are available to measure the fair value less
costs of disposal. Therefore, an estimate is derived indi-
rectly and is based on net present value techniques uti-
lizing post-tax cash flows and discount rates. In the lim-
ited cases where the value-in-use method would be
applied, net present value techniques would be applied
using pre-tax cash flows and discount rates.
Fair value less costs of disposal reflects estimates of
assumptions that market participants would be expected
to use when pricing the asset or CGU, and for this pur-
pose, management considers the range of economic
conditions that are expected to exist over the remaining
useful life of the asset. These valuations are classified
as “Level 3” in the fair value hierarchy.
The estimates used in calculating the net present val-
ues are highly sensitive and depend on assumptions spe-
cific to the nature of the Group’s activities with regard
to:

Notes to the Novartis Groupconsolidatedfinancial statements
F-9
• Amount and timing of projected future cash flows
• Sales forecasts
• Actions of competitors (launch of competing products,
marketing initiatives, etc.)
• Sales erosion rates after the end of patent or other
intellectual property rights protection, and timing of the
entry of generic competition
• Outcome of research and development activities (com-
pound ecacy, results of clinical trials, etc.)
• Amount and timing of projected costs to develop IPR&D
into commercially viable products
• Profit margins
• Probability of obtaining regulatory approval
• Future tax rate
• Appropriate terminal growth rate
• Appropriate discount rate
Generally, for intangible assets with a definite useful life,
Novartis uses cash flow projections for the whole useful
life of these assets. For goodwill, Novartis generally uti-
lizes cash flow projections for a three-year period based
on management forecasts, with a terminal value based
on cash flow projections usually in line with inflation rates
for later periods.
Probability-weighted scenarios are typically used.
Discount rates used consider the Group’s estimated
weighted average cost of capital, adjusted for specific
asset, country and currency risks associated with cash
flow projections, to approximate the discount rate that
market participants would use to value the asset.
Due to the above factors, actual cash flows and val-
ues could vary significantly from forecasted future cash
flows and related values derived using discounting tech-
niques.
Cash and cash equivalents
Cash and cash equivalents include highly liquid invest-
ments with original maturities of three months or less,
which are readily convertible to known amounts of cash.
Bank overdrafts are presented within current financial
debts on the consolidated balance sheet.
Marketable securities, commodities
and non-current financial assets
Commodities, which include gold bullion or coins, are
valued at the lower of cost or fair value using current
market prices. The changes in fair value below cost are
immediately recorded in “Other financial income and
expense.”
Marketable securities are financial assets held for
short-term purposes which are principally traded in liq-
uid markets and are classified within current assets on
the consolidated balance sheet. The financial impacts
related to these financial assets are recorded in “Other
financial income and expense” in the consolidated
income statement. Non-current financial assets held for
long-term strategic purposes are classified within
non-current assets on the consolidated balance sheet.
The financial impacts related to these financial assets
are recorded in “Other income” and “Other expense” in
the consolidated income statement.
Marketable securities and non-current financial
assets are initially recorded at fair value on their trade
date, which is dierent from the settlement date when
the transaction is ultimately eected. Quoted securities
are remeasured at each reporting date to fair value based
on current market prices. If the market for a financial
asset is not active or no market is available, fair values
are established using valuation techniques. The major-
ity of non-quoted investments are initially valued at fair
value through the purchase price established between
a willing buyer and seller. Non-quoted investments are
subsequently adjusted based on values derived from dis
-
counted cash flow analysis or other pricing models.
These investment values are classified as “Level 3” in
the fair value hierarchy.
The Group classifies and accounts for its marketable
securities and non-current financial assets in the follow-
ing categories:
• Debt securities are valued at fair value through other
comprehensive income with subsequent recycling into
the consolidated income statement, as they meet both
the “solely payment of principal and interest” and the
business model criteria. Unrealized gains and losses,
except exchange gains and losses, are recorded as a
fair value adjustment in the consolidated statement of
comprehensive income. They are recognized in the
consolidated income statement when the debt instru-
ment is sold, at which time the gain is transferred to
“Other financial income and expense.” Exchange gains
and losses related to debt instruments are immediately
recognized in the consolidated income statement in
“Other financial income and expense.”
• Fund investments and equity securities of the Novartis
Venture Fund are valued at fair value through profit and
loss (FVPL). Unrealized gains and losses, including
exchange gains and losses, are recognized in the con-
solidated income statement in “Other income” for gains
and “Other expense” for losses.
• Equity securities held as strategic investments, typi-
cally held outside of the Novartis Venture Fund, are
generally designated at the date of acquisition as finan-
cial assets valued at fair value through other compre-
hensive income with no subsequent recycling through
profit and loss. Unrealized gains and losses, including
exchange gains and losses, are recorded as a fair value
adjustment in the consolidated statement of compre-
hensive income. They are reclassified to retained earn-
ings when the equity security is sold. If these equity
securities are not designated at the date of acquisition
as financial assets valued at fair value through other
comprehensive income, they are valued at FVPL, as
described above.
• Other non-current financial assets, such as loans and
long-term receivables from customers, advances and
other deposits, are valued at amortized cost, which
reflects the time value of money less any allowances
for expected credit losses.
The Group assesses on a forward-looking basis the
expected credit losses associated with its debt securi-
ties valued at fair value through other comprehensive

Notes to the Novartis Groupconsolidatedfinancial statements
F-10
income. Impairments on debt securities are recorded in
“Other financial income and expense.”
For other financial assets valued at amortized cost,
impairments, which are based on their expected credit
losses, and exchange rate losses are included in “Other
expense” in the consolidated income statement.
Exchange rate gains and interest income, using the eec-
tive interest rate method, are included in “Other income”
or “Other financial income” in the consolidated income
statement, depending on the nature of the item.
Derivative financial instruments
Derivative financial instruments are initially recognized
in the balance sheet at fair value and are remeasured to
their current fair value at the end of each subsequent
reporting period. The valuation of a forward exchange
rate contract is based on the discounted cash flow
model, using interest rate curves and forward rates at
the reporting date as observable inputs.
Options are valued based on a modified Black-
Scholes model using volatility and exercise prices as
major observable inputs.
The Group enters into certain derivative financial
instruments for the purpose of hedging to reduce the
volatility in the Group’s performance due to the exposure
to various business-related risks. The risk mitigation is
obtained because the derivative’s value or cash flows
are expected, wholly or partly, to oset changes in the
value or cash flows of the recognized assets or liabilities.
The overall strategy is aiming to mitigate the currency
and interest rate risk of positions that are contractually
agreed, and to partially mitigate the exposure risk of
selected anticipated transactions.
Certain derivative financial instruments meet the
criteria for hedge accounting treatment. A prerequisite
for obtaining this accounting-hedge relationship is exten-
sive documentation on inception and proving on a regu-
lar basis that the economic hedge is eective for account-
ing purposes. Other derivative financial instruments do
not meet the criteria to qualify for hedge accounting or
are not designated in a hedge relationship. Changes in
the fair value of these derivative instruments are recog-
nized immediately in “Other financial income and
expense” in the consolidated income statement.
In addition, the Group has designated certain long-
term debt components as hedges of the translation risk
arising on certain net investments in foreign operations.
On consolidation, foreign currency dierences arising
on long-term debt designated as net investment hedges
of a foreign operation are recognized in other compre-
hensive income and accumulated in currency translation
eects, to the extent that the hedge is eective. The for-
eign currency dierences arising from hedge ineective-
ness are recognized in the income statement in “Other
financial income and expense.”
When a hedged net investment is disposed of, the
proportionate portion of the cumulative amount recog-
nized in equity in relation to the hedged net investment
is transferred to the consolidated income statement as
an adjustment to the gain or loss on disposal.
Inventories
Inventory is valued at the lower of acquisition or produc-
tion cost determined on a first-in, first-out basis and net
realizable value. This value is used for the “Cost of goods
sold” in the consolidated income statement. Unsaleable
inventory is fully written o in the consolidated income
statement under “Cost of goods sold.”
Trade receivables
Trade receivables are initially recognized at their invoiced
amounts, including any related sales taxes less adjust-
ments for estimated revenue deductions such as rebates,
chargebacks and cash discounts.
Provisions for doubtful trade receivables are estab-
lished using a forward-looking expected credit loss
model (ECL), which includes possible default events on
the trade receivables over the entire holding period of
the trade receivable. These provisions represent the dif-
ference between the trade receivable’s carrying amount
in the consolidated balance sheet and the estimated col-
lectible amount. Charges for doubtful trade receivables
are recorded as marketing and selling costs recognized
in the consolidated income statement within “Selling,
general and administration” expenses.
Legal and environmental liabilities
Novartis and its subsidiaries are subject to contingen-
cies arising in the ordinary course of business, such as
patent litigation, environmental remediation liabilities and
other product-related and commercial litigation, and gov-
ernmental investigations and proceedings. A provision
is recorded when there is a probable outflow of resources
for which a reliable estimate can be made of the outcome
of the legal or other disputes against the subsidiary.
Contingent consideration
In the acquisition or divestment of a business, it is nec-
essary to recognize contingent future amounts due to
previous owners, representing contractually defined
potential amounts as a liability or an asset. Usually for
Novartis, these are linked to milestone or royalty pay-
ments related to certain assets and are recognized as a
financial liability or financial asset at fair value, which is
then remeasured at each subsequent reporting date.
These estimations typically depend on factors such as
technical milestones or market performance, and are
adjusted for the probability of their likelihood of payment,
and are appropriately discounted to reflect the impact
of time.
Changes in the fair value of contingent consideration
liabilities in subsequent periods are recognized in the
consolidated income statement in “Cost of goods sold”
for currently marketed products and in “Research and
development” for IPR&D. Changes in contingent consid-
eration assets are recognized in “Other income” or
“Other expense,” depending on their nature.

Notes to the Novartis Groupconsolidatedfinancial statements
F-11
The eect of unwinding the discount over time is rec-
ognized for contingent consideration liabilities in “Inter-
est expense” and for contingent consideration assets as
interest income recognized in the consolidated income
statement within “Other financial income and expense.”
Defined benefit pension plans
andotherpost-employment benefits
The liability in respect of defined benefit pension plans
and other post-employment benefits is the defined ben-
efit obligation calculated annually by independent actu-
aries using the projected unit credit method. The current
service cost for such post- employment benefit plans is
included in the personnel expenses of the various func-
tions in which employees are employed, while the net
interest on the net defined benefit liability or asset is
recognized as “Other expense” or “Other income.”
Treasury shares
Treasury shares are initially recorded at fair value on their
trade date, which is dierent from the settlement date,
when the transaction is ultimately eected. Treasury
shares are deducted from consolidated equity at their
nominal value of CHF 0.50 per share. Dierences
between the nominal amount and the transaction price
on purchases or sales of treasury shares with third par-
ties, or the value of services received for the shares allo-
cated to employees as part of share-based compensa-
tion arrangements, are recorded in “Retained earnings”
in the consolidated statement of changes in equity.
Revenue recognition
Revenue on the sale of Novartis Group products and ser
-
vices, which is recorded as “Net sales to third parties” in
the consolidated income statement, is recognized when
a contractual promise to a customer (performance obli-
gation) has been fulfilled by transferring control over the
promised goods and services to the customer, substan-
tially all of which is at the point in time of shipment to or
receipt of the products by the customer or when the ser-
vices are performed. If contracts contain customer
acceptance provisions, revenue is recognized upon the
satisfaction of the acceptance criteria. If a contract con-
tains more than one performance obligation, the consid-
eration is allocated based on the standalone selling price
of each performance obligation. The amount of revenue
recognized is based on the consideration Novartis
expects to receive in exchange for its goods and ser-
vices, when it is highly probable that a significant rever-
sal will not occur.
The consideration Novartis receives in exchange for
its goods or services may be fixed or variable. Variable
consideration is recognized when it is highly probable
that a significant reversal will not occur. The most com-
mon elements of variable consideration are listed below.
• Rebates and discounts granted to wholesalers, retail-
ers, government agencies (including US Medicaid and
US Federal Medicare programs), government
supported healthcare systems, private health systems,
pharmacy benefit managers, managed healthcare
organizations, purchasing organizations and other
direct and indirect customers, as well as chargebacks
are provisioned and recorded as revenue deductions
at the time the related revenues are recorded, or when
the incentives are oered. These rebates and dis-
counts, applied using provision rates, are estimated
based on the terms and conditions in the individual
states, plans and customer agreements, historical
experience, product sales and growth rate, population
growth, product pricing including inflation impacts, the
mix of contracts and products, the level of inventory in
the distribution channel, regulations, contracts, chan-
nels and payers, as appropriate to the individual rebate
and discount arrangements.
• Refunds granted to healthcare providers under
innovative pay-for-performance agreements (i.e. out-
come based arrangements) are provisioned and
recorded as a revenue deduction at the time the related
sales are recorded. They are calculated on the basis
of historical experience and clinical data available for
the product, as well as specific terms of the individual
agreements. In cases where historical experience and
clinical data are not sucient for a reliable estimation
of the outcome, revenue recognition is deferred until
the uncertainty is resolved, until such history is avail-
able or the period when the refund right has expired.
The provisions for revenue deductions under the
innovative pay-for-performance agreements are
adjusted periodically based on established processes
and actual experience, including the products actual
outcomes achieved compared with the anticipated pre-
defined targets.
• Cash discounts are oered to customers to encourage
prompt payment and are provisioned and recorded as
revenue deductions at the time the related sales are
recorded.
• Shelf stock adjustments are generally granted to cus-
tomers, primarily of the Sandoz Division, to cover the
inventory held by them at the time a price decline
becomes eective. Revenue deduction provisions for
shelf stock adjustments are recorded when the price
decline is anticipated, based on the impact of the price
decline on the customer’s estimated inventory levels.
• Sales returns provisions are recognized and recorded
as revenue deductions when there is historical expe-
rience of Novartis agreeing to customer returns and
Novartis can reasonably estimate expected future
returns. In doing so, the estimated rate of return is
applied, determined on the basis of historical experi-
ence of customer returns and considering any other
relevant factors. This is applied to the amounts invoiced,
also considering the amount of returned products to
be destroyed versus products that can be placed back
in inventory for resale. Where shipments are made on
a resale or return basis, without sucient historical
experience for estimating sales returns, revenue is only
recorded when there is evidence of consumption or
when the right of return has expired.
Net sales to third parties and provisions for revenue
deductions are adjusted periodically to reflect experi-
ence and to reflect actual amounts as rebates, refunds,

Notes to the Novartis Groupconsolidatedfinancial statements
F-12
discounts and returns are processed. There is often a
time lag between recording of revenue deductions and
the final accounting for them. The provision represents
estimates of the related obligations, requiring the use of
judgment when estimating the eect of these revenue
deductions.
“Other revenue” includes income from profit-sharing
arrangements with our collaboration partners, and roy-
alty and milestone income from the out-licensing of intel-
lectual property when Novartis retains an interest in the
intellectual property through a license. Royalty income
earned from a license is recognized when the underly-
ing sales have occurred. Milestone income is recognized
at the point in time when it is highly probable that the rel-
evant milestone event criteria are met, and the risk of
reversal of revenue recognition is remote. “Other reve-
nue” also includes revenue from activities such as man-
ufacturing or other services rendered, to the extent such
revenue is not recorded under net sales to third parties,
and is recognized when control transfers to the third
party and our performance obligations are satisfied.
Research and development
Internal research and development (R&D) costs are fully
charged to “Research and development” in the consol-
idated income statement in the period in which they are
incurred. The Group considers that regulatory and other
uncertainties inherent in the development of new prod-
ucts preclude the capitalization of internal development
expenses as an intangible asset until marketing approval
from a regulatory authority is obtained in a major market
such as the United States, the European Union, Switzer-
land or Japan.
Payments made to third parties, such as contract
research and development organizations in compensa-
tion for subcontracted R&D, that are deemed not to
transfer intellectual property to Novartis are expensed
as internal R&D expenses in the period in which they are
incurred. Such payments are only capitalized if they meet
the criteria for recognition of an internally generated
intangible asset, usually when marketing approval has
been received from a regulatory authority in a major mar-
ket.
Payments made to third parties to in-license or
acquire intellectual property rights, compounds and
products, including initial upfront and subsequent mile-
stone payments, are capitalized, as are payments for
other assets, such as technologies to be used in R&D
activities. If additional payments are made to the origi-
nator company to continue performing R&D activities, an
evaluation is made as to the nature of the payments. Such
additional payments will be expensed if they are deemed
to be compensation for subcontracted R&D services not
resulting in an additional transfer of intellectual property
rights to Novartis. Such additional payments will be cap-
italized if they are deemed to be compensation for the
transfer to Novartis of additional intellectual property
developed at the risk of the originator company. Subse-
quent internal R&D costs in relation to IPR&D and other
assets are expensed, since the technical feasibility of
the internal R&D activity can only be demonstrated by
the receipt of marketing approval for a related product
from a regulatory authority in a major market.
Costs for post-approval studies performed to sup-
port the continued registration of a marketed product
are recognized as marketing expenses. Costs for activ-
ities that are required by regulatory authorities as a con-
dition for obtaining marketing approval in a major market
are capitalized and recognized as currently marketed
products.
Inventory produced ahead of regulatory approval is
fully provisioned, and the charge is included in “Other
expense” in the consolidated income statement, as its
ultimate use cannot be assured. If this inventory can sub-
sequently be sold, the provision is released to “Other
income” in the consolidated income statement, either on
approval by the appropriate regulatory authority or,
exceptionally in Europe, on recommendation by the
Committee for Medicinal Products for Human Use
(CHMP), if approval is virtually certain.
Share-based compensation
Vested Novartis shares and American Depositary
Receipts (ADRs) that are granted as compensation are
valued at their market value on the grant date and are
immediately expensed in the consolidated income state-
ment.
The fair values of unvested restricted shares (RSs),
restricted share units (RSUs) and performance share
units (PSUs) in Novartis shares and ADRs granted to
employees as compensation are recognized as an
expense over the related vesting period. The expense
recorded in the consolidated income statement is
included in the personnel expenses of the various func-
tions in which the employees are employed.
Unvested restricted shares, restricted ADRs and
RSUs are only conditional on the provision of services
by the plan participant during the vesting period. They
are valued at fair value on the grant date. As RSUs do
not entitle the holder to dividends, the fair value is based
on the Novartis share price at the grant date adjusted
for the net present value of the dividends expected to
be paid during the holding period. The fair value of these
grants, after making adjustments for assumptions related
to forfeiture during the vesting period, is expensed on a
straight-line basis over the respective vesting period.
PSUs are subject to the achievement of certain per-
formance criteria during the vesting period and require
plan participants to provide services during this period.
The following paragraphs provide an overview of the
accounting policies for the share-based compensation
plan that grant PSUs.
For PSUs that are subject to performance criteria
based on Novartis internal performance metrics and that
are conditional on the provision of service by plan par-
ticipants during the vesting period, the expense is rec-
ognized on a straight-line basis over the vesting period,
and is determined based on assumptions concerning the
expected performance against the internal performance
metrics throughout the vesting period. The assumptions
are based on the Group’s targets for those performance
metrics, and the expected forfeitures due to plan partic-
ipants not meeting their service conditions. The

Notes to the Novartis Groupconsolidatedfinancial statements
F-13
assumptions are periodically adjusted over the vesting
period. Any change in estimates for past services is
recorded immediately as an expense or income in the
consolidated income statement, and amounts for the
remaining vesting period are expensed on a straight-line
basis. As a result, at the end of the vesting period, the
charge during the entire vesting period represents the
amount that will finally vest. The number of equity instru-
ments that finally vest is determined at the vesting date.
For PSUs that are subject to performance criteria
based on variables that can be observed in the market,
which for Novartis plans is the Novartis total shareholder
return (TSR) relative to a specific peer group of compa-
nies over the vesting period, and that are conditional on
the provision of services by the plan participants during
the vesting period, the expense is recognized on a
straight-line basis over the vesting period, and is deter-
mined based on the total fair value of the grant over the
vesting period. IFRS requires that these variables that
can be observed in the market are taken into account in
determining the fair value of the PSUs at the grant date.
Novartis determined the fair value of these PSUs at the
date of grant using a Monte Carlo simulation model.
Adjustments to the number of equity instruments granted
are only made if a plan participant does not fulfill the ser-
vice conditions.
For PSUs granted under plans that are subject to both
performance criteria based on Novartis internal perfor-
mance metrics and Novartis TSR relative to a specific
peer group of companies over the vesting period and
that are conditional on the provision of service by plan
participants during the vesting period, the expense is
recognized on a straight-line basis over the vesting
period, and is determined based on a bifurcation into the
components based on the performance criteria related
to Novartis internal performance metrics and TSR, as
described in the paragraphs above.
Measuring the fair values of PSUs granted that
include TSR performance criteria requires use of esti-
mates. The Monte Carlo simulation used to determine
the fair value of the PSUs TSR performance criteria
requires the probability of factors related to uncertain
future events; the term of the award; the grant price of
underlying shares or ADRs; expected volatilities; the
expected correlation matrix of the underlying equity
instruments with those of the peer group of companies;
and the risk-free interest rate as input parameters.
If a plan participant leaves Novartis for reasons other
than retirement, disability or death, then unvested
restricted shares, restricted ADRs, RSUs and PSUs are
forfeited, unless determined otherwise by the provision
of the plan rules or by the Compensation Committee of
the Novartis Board of Directors, for example, in connec-
tion with a reorganization or divestment.
Government grants
Grants from governments or similar organizations are
recognized at their fair value when there is reasonable
assurance that the grant will be received and the Group
will comply with all attached conditions.
Government grants received to compensate costs
are deferred and recognized in the consolidated income
statement over the period necessary to match them
against the related costs that they are intended to com-
pensate.
The accounting policy for property, plant and equip-
ment describes the treatment of any related grants.
Restructuring charges
Restructuring provisions are recognized for the direct
expenditure arising from the restructuring, where the
plans are suciently detailed and where appropriate
communication to those aected has been made.
Charges to increase restructuring provisions are
included in “Other expense” in the consolidated income
statements.
Healthcare contributions
Healthcare cost contribution levies and fees under gov-
ernmental programs that require the Group to contrib-
ute to a country’s healthcare costs, other than programs
described in “Revenue recognition” in this Note 1, are
recognized in “Other expense” in the consolidated
income statement. Provisions for healthcare cost con-
tributions are adjusted to the actual amounts levied. The
provision represents estimates of the related obligations,
requiring the use of judgment when estimating the eect
of these healthcare cost contributions.
Income taxes
Income taxes comprise current income taxes and
deferred income taxes and are recognized in the same
periods as the revenues and expenses to which they
relate. Income taxes include interest and penalties
incurred during the period, insofar as they are consid-
ered an income tax. Income taxes related to items rec-
ognized directly to other comprehensive income or to
equity are recognized together with the corresponding
item, to which the income tax is attributable, directly in
other comprehensive income or in equity.
Deferred income taxes are determined using the
comprehensive liability method and are calculated on
the temporary dierences that arise between the tax
base of an asset or liability and its carrying value for
financial reporting purposes, except for those temporary
dierences related to investments in subsidiaries and
associated companies, where the timing of their rever-
sal can be controlled and it is probable that the dier-
ence will not reverse in the foreseeable future. Since the
retained earnings are reinvested, withholding or other
taxes on eventual distribution of a subsidiary’s retained
earnings are only recognized when a dividend is declared
or has been planned. Furthermore, deferred income
taxes are recognized for the net tax eects of net oper-
ating loss carryforwards and tax credits.
The carrying amount of deferred tax assets is
reduced to the extent that it is not probable that su-
cient taxable profits will be available to enable all or part
of the asset to be recovered. In evaluating our ability to
recover our deferred tax assets in the jurisdiction from

Notes to the Novartis Groupconsolidatedfinancial statements
F-14
which they arise, we consider all available positive and
negative evidence, including scheduled reversals of
deferred tax liabilities, projected future taxable income,
tax-planning strategies, and results of recent operations.
The estimated amounts for current and deferred tax
assets or liabilities, including amounts related to any
uncertain tax positions, are based on applicable tax law
and regulations in the various tax jurisdictions, in which
the Group operates, which are subject to interpretations
based on currently known facts and circumstances.
Tax returns are based on an interpretation of tax laws
and regulations, and reflect estimates based on these
judgments and interpretations. The tax returns are sub-
ject to examination by the competent taxing authorities,
which may result in an assessment being made requir-
ing payments of additional tax, interest or penalties.
The calculation of income tax assets and liabilities
involves dealing with uncertainties in the application of
complex tax laws and regulations in a multitude of juris-
dictions across our global operations. As a result, inher-
ent uncertainties exist in the estimates of the tax posi-
tions. Tax liabilities for uncertain tax provisions are
recognized on the consolidated balance sheets within
current income tax liabilities.
Impact of new IFRS standards,
amendments and interpretations in
2022
There were no new IFRS standards adopted by the
Group in 2022. In addition, new IFRS amendments or
interpretations that became eective in 2022 did not
have a material impact to the Group’s consolidated finan-
cial statements.
Based on the Group’s assessment, there are no IFRS
standards, amendments or interpretations not yet eec-
tive in 2022 that would be expected to have a material
impact on the Group’s consolidated financial statements.
Impact of adopting significant new
IFRS standard in 2021
The following new IFRS standard has been adopted by
Novartis from January 1, 2021:
Interest Rate Benchmark Reform – Phase 2,
Amendments to IFRS 9, IAS 39, IFRS 7, IFRS 4 and
IFRS 16 (Interest Benchmark Reform Amendments)
Interest Benchmark Reform Amendments became eec-
tive from January 1, 2021. These amendments address
issues that might aect financial reporting when an exist-
ing interest rate benchmark (i.e. Interbank oered rate –
IBOR) is replaced with an alternative benchmark inter-
est rate. The eects of interest rate benchmark reform
on the Group’s financial instruments and risk manage-
ment strategies did not have a material impact on the
Group’s consolidated financial statements.
Impact of adopting significant new
IFRS standard in 2020
The following new IFRS standard has been adopted by
Novartis from January 1, 2020:
IFRS 3 Business Combinations amendments
The IASB issued amendments to IFRS 3 Business Com-
binations that revised the definition of a business, which
assist entities with the evaluation of when an asset or
group of assets acquired should be considered a busi-
ness. This amended standard has been applied to trans-
actions entered into on or after January 1, 2020. The
amended standard allows an entity to apply an optional
concentration test, on a transaction-by-transaction
basis, to evaluate whether substantially all of the fair
value of the gross assets acquired is concentrated in a
single identifiable asset or group of similar identifiable
assets. If this optional concentration test is met, the set
of activities and assets is determined not to be a busi-
ness. The adoption of this amended standard on Janu-
ary 1, 2020, did not have a significant impact on our con-
solidated financial statements and is not expected to
have a significant impact in future periods. However, this
will depend on the facts and circumstances of future
transactions and if the Group decides to apply the
optional concentration test in the assessment of whether
an acquired set of activities and assets is or is not a busi-
ness.

Notes to the Novartis Groupconsolidatedfinancial statements
F-15
2. Significant transactions
The Group applied the acquisition method of account-
ing for businesses acquired, and did not elect to apply
the optional concentration test to account for acquired
business as an asset separately acquired.
Significant transactions in 2022
Innovative Medicines – acquisition of Gyroscope
Therapeutics Holdings plc
On December 22, 2021, Novartis entered into an agree-
ment to acquire all outstanding shares of Gyroscope
Therapeutics Holdings plc (Gyroscope), a UK-based
ocular gene therapy company. Gyroscope focuses on
the discovery and development of gene therapy treat-
ments for retinal indications. The purchase price con-
sisted of a cash payment of USD 0.8 billion, subject to
certain customary purchase price adjustments, and
potential additional milestone payments of up to USD 0.7
billion, which Gyroscope shareholders are eligible to
receive upon achievement of specified milestones. The
acquisition closed on February 17, 2022.
The fair value of the total purchase consideration was
USD 1.0 billion. The amount consisted of an upfront cash
payment of USD 0.8 billion (including customary pur-
chase price adjustments) and the fair value of contingent
consideration of USD 0.2 billion, which Gyroscope share-
holders are eligible to receive upon achievement of spec
-
ified milestones. The purchase price allocation resulted
in net identifiable assets of USD 0.9 billion, consisting
primarily of intangible assets of USD 1.1 billion and net
deferred tax liabilities of USD 0.2 billion. Goodwill
amounted to USD 0.1 billion.
The results of operations since the date of acquisi-
tion are not material.
Significant transactions in 2021
Sandoz – acquisition of GSK’s cephalosporin
antibiotics business
On February 10, 2021, Sandoz entered into an agreement
with certain subsidiaries of GlaxoSmithKline plc (GSK)
for the acquisition of the GSK’s cephalosporin antibiot-
ics business.
Under the agreement, Sandoz acquired the global
rights to three established brands (Zinnat
®
, Zinacef
®
and
Fortum
®
) in more than 100 markets. It excluded the rights
in the US, Australia and Germany to certain of those
brands, which were previously divested by GSK, and the
rights in India, Pakistan, Egypt, Japan (to certain of the
brands) and China, which will be retained by GSK. The
transaction closed on October 8, 2021.
The purchase price consisted of a USD 350 million
upfront payment paid at closing and potential milestone
payments up to USD 150 million, which GSK will be eli-
gible to receive upon the achievement of certain annual
sales milestones for the portfolio.
The fair value of the total purchase consideration was
USD 415 million. The amount consisted of a payment of
USD 351 million, including purchase price adjustments,
and the fair value of contingent consideration of USD 64
million, which GSK is eligible to receive upon the achieve-
ment of specified milestones. The purchase price allo-
cation resulted in net identifiable assets of USD 308 mil-
lion, consisting of USD 292 million intangible assets and
USD 16 million deferred tax assets. Goodwill amounted
to USD 107 million.
The 2021 results of operations since the date of
acquisition were not material.
Corporate – divestment of the investment in Roche
Holding AG
On November 3, 2021, Novartis entered into a Share
Repurchase Agreement with Roche Holding AG under
which Novartis agreed to sell 53.3 million (approximately
33.3%) bearer shares of Roche Holding AG voting shares
in a bilateral transaction to Roche Holding AG for a total
consideration of USD 20.7 billion. As a result, Novartis
discontinued the use of equity method accounting start-
ing from November 3, 2021.
The transaction closed on December 6, 2021.
Novartis realized a gain of USD 14.6 billion, recorded in
income from associated companies.
Significant transactions in 2020
Innovative Medicines – acquisition of
TheMedicines Company
On November 23, 2019, Novartis entered into an agree-
ment and plan of merger (“the Merger Agreement”) with
The Medicines Company, a US-based pharmaceutical
company headquartered in Parsippany, New Jersey,
USA. Pursuant to the Merger Agreement, on December
5, 2019, Novartis, through a subsidiary, commenced a
tender oer to acquire all outstanding shares of The
Medicines Company for USD 85 per share, or a total con-
sideration of approximately USD 9.6 billion in cash on a
fully diluted basis, including the equivalent share value
related to The Medicines Company’s convertible notes,
in accordance with their terms. The tender oer expired
on January 3, 2020, and on January 6, 2020, the acquir-
ing subsidiary merged with and into The Medicines Com-
pany, resulting in The Medicines Company becoming an
indirect wholly owned subsidiary of Novartis. Novartis
financed the transaction through available cash, and
short- and long-term borrowings.
The Medicines Company is focused on the develop-
ment of inclisiran, a potentially first-in-class, twice yearly
therapy that allows administration during patients’ rou-
tine visits to their healthcare professionals and will poten-
tially contribute to improved patient adherence and sus-
tained lower LDLC levels.
The fair value of the total purchase consideration was
USD 9.6 billion. The purchase price allocation resulted
in net identifiable assets of approximately USD 7.1 billion,
consisting of USD 8.5 billion intangible assets, USD 1.4

Notes to the Novartis Groupconsolidatedfinancial statements
F-16
billion net deferred tax liabilities and goodwill of approx-
imately USD 2.5 billion.
The 2020 results of operations since the date of
acquisition were not material.
Sandoz – acquisition of the Japanese business of
Aspen Global Incorporated
On November 11, 2019, Sandoz entered into an agree-
ment for the acquisition of the Japanese business of
Aspen Global Incorporated (AGI), a wholly owned sub-
sidiary of Aspen Pharmacare Holdings Limited. Under
the agreement, Sandoz acquired the shares in Aspen
Japan K.K. and associated assets held by AGI. The trans-
action closed on January 31, 2020.
Aspen’s portfolio in Japan consisted of o-patent
medicines with a focus on anesthetics and specialty
brands. The acquisition will enable Sandoz to expand its
presence in the third-largest worldwide generics mar-
ketplace.
The purchase price consisted of EUR 274 million
(USD 303 million) upfront payment, less customary pur-
chase price adjustment of EUR 27 million (USD 30 mil-
lion), plus potential milestone payments of up to EUR 70
million (USD 77 million), which AGI is eligible to receive
upon the achievement of specified milestones.
The fair value of the total purchase consideration was
EUR 294 million (USD 324 million). The amount consisted
of a cash payment of EUR 247 million (USD 273 million)
and the fair value of contingent consideration of EUR 47
million (USD 51 million), which AGI is eligible to receive
upon the achievement of specified milestones. The pur-
chase price allocation resulted in net identifiable assets
of USD 238 million, consisting of USD 196 million intan-
gible assets, USD 26 million other net assets and USD
16 million net deferred tax assets. Goodwill amounted to
USD 86 million.
The 2020 results of operations since the date of
acquisition were not material.
Sandoz – retention of US dermatology business
and generic US oral solids portfolio, previously
planned to be divested
On September 6, 2018, Novartis announced that it
entered into a stock and asset purchase agreement
(SAPA) with Aurobindo Pharma USA Inc. (Aurobindo) for
the sale of selected portions of its Sandoz US portfolio,
specifically the Sandoz US dermatology business and
generic US oral solids portfolio, for USD 0.8 billion in
cash and potential earnouts. The closing was conditional
on obtaining regulatory approval.
In March 2020, Novartis took the decision to retain
the Sandoz US generic oral solids and dermatology busi-
nesses and on April 2, 2020 entered into a mutual agree-
ment with Aurobindo to terminate the transaction. The
decision was taken as approval from the US Federal
Trade Commission for the transaction was not obtained
within the agreed timelines.
The cumulative amount of the depreciation on prop-
erty, plant and equipment (USD 38 million) and amorti-
zation on intangible assets (USD 102 million) not recorded
in the consolidated income statement since the date of
classification as held for sale was recognized in the con-
solidated income statement in the first quarter of 2020.
In addition, an impairment of currently marketed prod-
ucts of USD 42 million was recognized in the first quar-
ter of 2020 consolidated income statement.
As at March 31, 2020, the assets and liabilities of the
Sandoz US generic oral solids and dermatology busi-
nesses were reclassified out of assets and liabilities of
disposal group held for sale. The prior year balance sheet
was not required to be restated.
There were no cumulative income or expenses
included in the other comprehensive income relating to
the disposal group.

Notes to the Novartis Groupconsolidatedfinancial statements
F-17
3. Segmentation of key figures 2022, 2021 and 2020
The businesses of Novartis are divided operationally on
a worldwide basis into two identified reporting segments:
Innovative Medicines and Sandoz. In addition, we sepa-
rately report Corporate activities.
Reporting segments are presented in a manner con-
sistent with the internal reporting to the chief operating
decision-maker, which is the Executive Committee of
Novartis. The reporting segments are managed sepa-
rately because they each research, develop, manufac-
ture, distribute and sell distinct products that require dif-
fering marketing strategies.
The Executive Committee of Novartis is responsible
for allocating resources and assessing the performance
of the reporting segments.
The reporting segments are as follows:
Innovative Medicines researches, develops, manu-
factures, distributes and sells patented pharmaceuticals.
Eective as of April 4, 2022, the Innovative Medicines
Division is organized in two commercial organizational
units: Innovative Medicines International and Innovative
Medicines US, and is focused on the core therapeutic
areas: cardiovascular; immunology; neuroscience; solid
tumors and hematology; as well as other promoted
brands (in the therapeutic areas of ophthalmology and
respiratory) and established brands. Prior to the
announcement on April 4, 2022, the Innovative Medicines
Division was organized into two global business units:
Novartis Oncology and Novartis Pharmaceuticals.
Sandoz develops, manufactures and markets finished
dosage form medicines as well as intermediary products
including active pharmaceutical ingredients. Sandoz is
organized globally into three franchises: Retail Generics,
Anti-Infectives and Biopharmaceuticals. In Retail Gener-
ics, Sandoz develops, manufactures and markets fin-
ished dosage forms of small molecule pharmaceuticals
for sale to third parties across a broad range of thera-
peutic areas, including finished dosage form of anti-in-
fectives sold to third parties. In Anti-Infectives, Sandoz
manufactures and supplies active pharmaceutical ingre-
dients and intermediates, mainly antibiotics, for internal
use by Retail Generics and for sale to third-party cus-
tomers. In Biopharmaceuticals, Sandoz develops, man-
ufactures and markets protein- or other biotechnolo-
gy-based products, including biosimilars, and provides
biotechnology manufacturing services to other compa-
nies.
Income and expenses relating to Corporate include
the costs of the Group headquarters and those of
corporate coordination functions in major countries. In
addition, Corporate includes other items of income and
expense that are not attributable to specific segments,
such as certain revenues from intellectual property
rights, certain expenses related to post-employment
benefits, environmental remediation liabilities, charitable
activities, donations and sponsorships. Usually, no allo-
cation of Corporate items is made to the segments. As
a result, Corporate assets and liabilities principally con-
sist of net debt (cash and cash equivalents, marketable
securities less financial debts), investments in associ-
ated companies, and current and deferred taxes and
non-segment-specific environmental remediation and
post-employment benefit liabilities.
Our divisions are supported by Novartis Institutes for
BioMedical Research, Global Drug Development, and the
Operations unit.
• The Novartis Institutes for BioMedical Research (NIBR)
conducts research activities for the Innovative
Medicines Division and also collaborates with Sandoz.
• The Global Drug Development organization oversees
all drug development activities for our Innovative
Medicines Division and collaborates with our Sandoz
Division on the development of its biosimilars portfo-
lio.
• The Operations unit, combines the Novartis Technical
Operations (NTO) and Customer & Technology Solu-
tions (CTS), following the internal reorganization
announced on April 4, 2022. The Operations unit man-
ages our manufacturing operations across our
Innovative Medicines and Sandoz Divisions, and deliv-
ers business support services across the Group, such
as information technology, real estate and facility ser-
vices and procurement.
The accounting policies mentioned in Note 1 are used in
the reporting of segment results. Inter-segmental sales
are made at amounts that are considered to approximate
arm’s length transactions. The Executive Committee of
Novartis evaluates segmental performance and allo-
cates resources among the segments based on a num-
ber of measures, including net sales to third parties,
operating income and net operating assets. Segment net
operating assets consist primarily of property, plant and
equipment; right-of-use assets; intangible assets; good-
will; inventories; and trade and other operating receiv-
ables less operating liabilities.

Notes to the Novartis Groupconsolidatedfinancial statements
F-18
Segmentation – consolidated income statements
Innovative Medicines Sandoz Corporate Group
(including eliminations)
(USD millions)
Net sales to third parties
Sales to other segments
–
–
Net sales
–
–
Other revenues
Cost of goods sold –
–
–
–
–
–
Gross profit
Selling, general and administration –
–
–
–
–
–
–
–
Research and development –
–
–
–
–
–
Other income
Other expense –
–
–
–
–
–
–
–
Operating income
–
–
(Loss)/income from associated companies –
–
–
Interest expense
–
–
Other financial income and expense
–
Income before taxes
Income taxes
–
–
Net income
Attributable to:
Shareholders of Novartis AG
6 955
24 021
Non-controlling interests
0
– 3
Included in net income are:
Interest income
Depreciation of property, plant and equipment –
–
–
–
–
–
–
–
Depreciation of right-of-use assets –
–
–
–
–
–
–
–
Amortization of intangible assets –
–
–
–
–
–
–
–
Impairment charges on property, plant and equipment, net –
–
–
–
–
–
Impairment of right-of-use assets –
–
Impairment charges on intangible assets, net –
–
–
–
–
–
–
–
Impairment charges and fair value
changes on financial assets, net –
–
–
–
Additions to restructuring provisions –
–
–
–
–
–
–
–
Equity-based compensation of Novartis equity plans –
–
–
–
–
–
–
–
1
Eliminations mainly relate to the elimination of sales to other segments and the corresponding cost of goods sold.

Notes to the Novartis Groupconsolidatedfinancial statements
F-19
Innovative Medicines Sandoz Corporate Group
(including eliminations)
(USD millions)
Net sales to third parties
Sales to other segments
–
–
Net sales
–
–
Other revenues
Cost of goods sold –
–
–
–
–
–
Gross profit
Selling, general and administration –
–
–
–
–
–
–
–
Research and development –
–
–
–
–
–
Other income
Other expense –
–
–
–
–
–
–
–
Operating income
–
–
Income from associated companies
Interest expense
–
–
Other financial income and expense
–
–
Income before taxes
Income taxes
–
–
Net income
Attributable to:
Shareholders of Novartis AG
24 021
8 072
Non-controlling interests
– 3
– 1
Included in net income are:
Interest income
Depreciation of property, plant and equipment –
–
–
–
–
–
–
–
Depreciation of right-of-use assets –
–
–
–
–
–
–
–
Amortization of intangible assets –
–
–
–
–
–
–
–
Impairment charges on property, plant and equipment, net –
–
–
–
–
–
–
Impairment charges on intangible assets, net –
–
–
–
–
–
–
–
Impairment charges and fair value
changes on financial assets, net
–
Additions to restructuring provisions –
–
–
–
–
–
–
–
Equity-based compensation of Novartis equity plans –
–
–
–
–
–
–
–
1
Eliminations mainly relate to the elimination of sales to other segments and the corresponding cost of goods sold.

Notes to the Novartis Groupconsolidatedfinancial statements
F-20
Segmentation – consolidated balance sheets
Innovative Medicines Sandoz Corporate Group
(including eliminations)
(USD millions)
Total assets
Total liabilities –
–
–
–
–
–
–
–
Total equity
Net debt
Net operating assets
–
–
Included in assets and liabilities are:
Total property, plant and equipment
Additions to property, plant
and equipment
Total right-of-use assets
Additions to right-of-use assets
Total goodwill and intangible assets
Additions to goodwill and
intangible assets
Total investment in associated
companies
Additions to investment in associated
companies
Cash and cash equivalents, marketable securities,
commodities, time deposits and derivative
financial instruments
Financial debts and derivative
financial instruments
Current income tax liabilities and deferred tax liabilities
1
Eliminations mainly relate to the elimination of intercompany receivables and payables to other segments and inventories
2
Note 29 provides additional disclosures related to net debt
3
Excluding the impact of business acquisitions
The following table shows countries that accounted for more than 5% of at least one of the respective Group totals,
as well as regional information for net sales to third parties for the years ended December 31, 2022, 2021 and 2020,
and for selected non-current assets for the years ended December 31, 2022 and 2021:
Net sales to third parties
Total of selected non-current assets
(USD millions)
Country
Switzerland
United States
France
Germany
China
Japan
Other
Group
Region
Europe
Americas
Asia/Africa/Australasia
Group
1
Net sales to third parties by location of customer
2
Total of property, plant and equipment; right-of-use assets; goodwill; intangible assets; investment in associated companies and other non-current assets excluding post-
employment benefit assets

Notes to the Novartis Groupconsolidatedfinancial statements
F-21
The Group’s largest, second-largest and third-largest cus-
tomers account for approximately 16%, 11% and 7% of net
sales to third parties, respectively (2021: 17%, 11% and 6%,
respectively; 2020: 17%, 11% and 6%, respectively). All seg-
ments had sales to these customers in 2022, 2021 and
2020.
The highest amounts of trade receivables outstanding
were for these same three customers and amounted to
approximately 16%, 14% and 7%, respectively, of the trade
receivables at December 31, 2022 (2021: 16%, 12% and
7%, respectively).
Segmentation – net sales to third parties
Net sales to third parties by region
1
Change
Change
(
(
to )
to )
USD m
USD m
USD
USD m
USD
Innovative Medicines
Europe
–
US
Asia/Africa/Australasia
–
Canada and Latin America
Total
–
Of which in Established Markets
–
Of which in Emerging Growth Markets
Sandoz
Europe
–
US
–
–
Asia/Africa/Australasia
–
Canada and Latin America
Total
–
Of which in Established Markets
–
–
Of which in Emerging Growth Markets
Group
Europe
–
US
Asia/Africa/Australasia
–
Canada and Latin America
Total
–
Of which in Established Markets
–
Of which in Emerging Growth Markets
1
Net sales to third parties by location of customer. Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan,
Australia and New Zealand.

Notes to the Novartis Groupconsolidatedfinancial statements
F-22
Innovative Medicines Division net sales to third parties by core therapeutic area; other
promoted brands; and established brands
Change
Change
( to
( to
)
)
USD m
USD m
USD
USD m
USD
Cardiovascular
Entresto
Leqvio
nm
nm
Total Cardiovascular
Immunology
Cosentyx
Xolair
–
Ilaris
Other
nm
nm
Total Immunology
Neuroscience
Gilenya
–
–
Zolgensma
Kesimpta
nm
Mayzent
Aimovig
Other
nm
Total Neuroscience
Solid Tumors
Tafinlar + Mekinist
Kisqali
Votrient
–
–
Lutathera
–
Piqray
Pluvicto
nm
nm
Tabrecta
Total Solid Tumors
Hematology
Promacta/Revolade
Tasigna
–
Jakavi
–
Kymriah
–
Adakveo
Scemblix
nm
nm
Other
–
Total Hematology
Change
Change
( to
( to
)
)
USD m
USD m
USD
USD m
USD
Other Promoted Brands
Lucentis
–
Xiidra
Ultibro Group
–
–
Beovu
–
Other respiratory
Total Other Promoted
Brands
–
Total Promoted
Brands
Established Brands
Sandostatin
–
–
Galvus Group
–
–
Gleevec/Glivec
–
–
Exforge Group
–
–
Diovan Group
–
–
Afinitor/Votubia
–
–
Voltaren/Cataflam
–
Zortress/Certican
–
–
Exjade/Jadenu
–
–
Neoral/Sandimmun(e)
–
–
Contract manufacturing
nm
Other
–
–
Total Established
Brands
–
–
Total division net
sales to third parties
–
1
Reclassified to reflect the new Innovative Medicines divisional structures announced
on April 4, 2022
2
Net sales to third parties reflect Xolair sales for all indications.
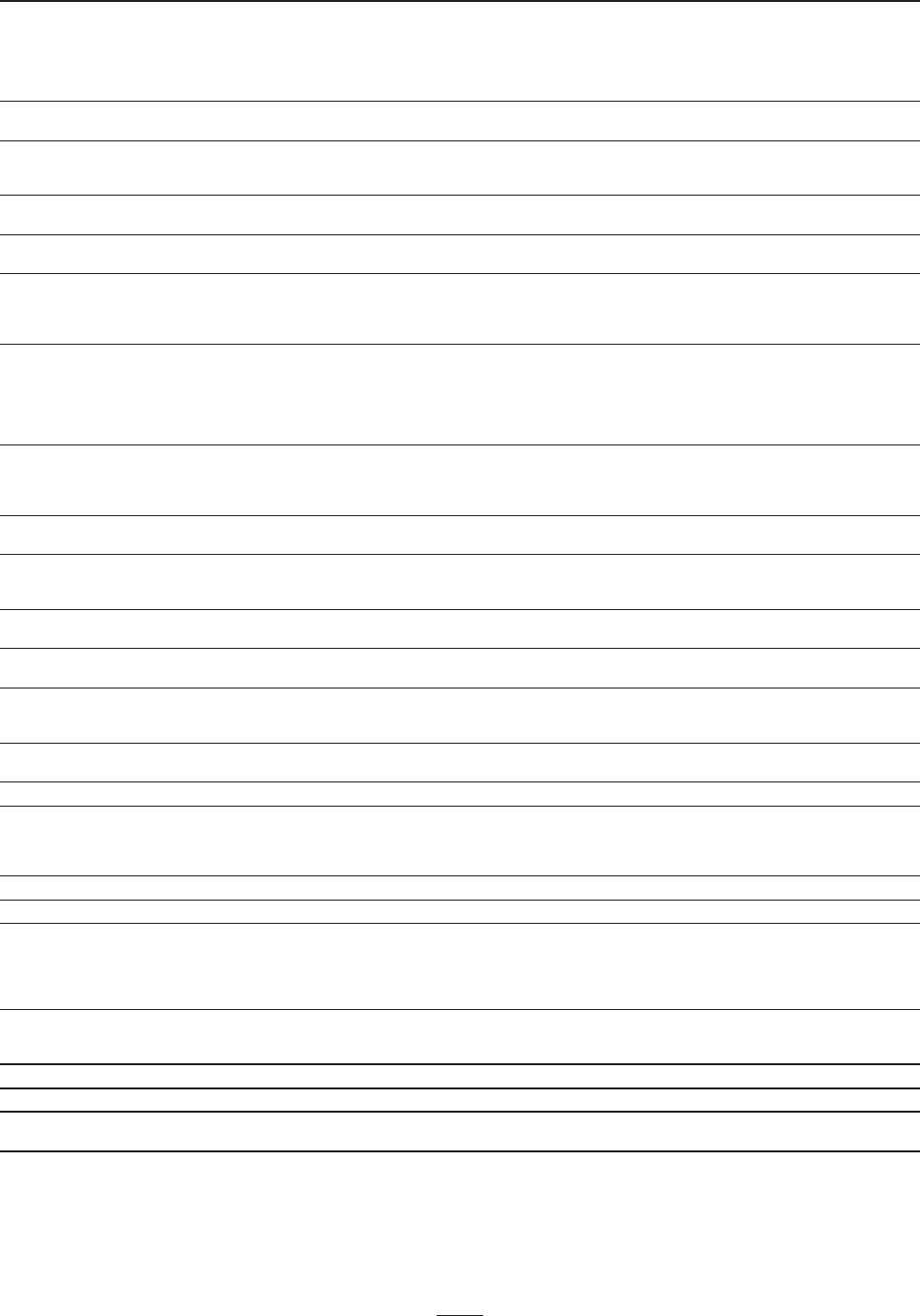
Notes to the Novartis Groupconsolidatedfinancial statements
F-23
Net sales to third parties of the top 20 Innovative Medicines Division brands in 2022
Brand classification by
therapeutic area, other
Rest of
promoted brands or US
world
Tot a l
Brands established brands Key indications USD m
USD m
USD m
Cosentyx Immunology Psoriasis (PsO),
ankylosing spondylitis
(AS), psoriatic arthritis
(PsA), non-radiographic
axial spondyloarthritis
(nr-axSPA)
Entresto Cardiovascular Chronic heart failure,
hypertension
Promacta/Revolade Hematology Immune
thrombocytopenia (ITP),
severe aplastic anemia (SAA)
Gilenya Neuroscience Relapsing multiple sclerosis
(RMS)
Tasigna Hematology Chronic myeloid leukemia
(CML)
Lucentis Other Promoted Age-related
Brands macular degeneration (AMD),
diabetic macular edema (DME),
retinal vein occlusion (RVO)
Tafinlar + Mekinist Solid Tumors BRAF V+ metastatic
adjuvant melanoma,
advanced non-small cell
lung cancer (NSCLC),
tumor agnostic with
BRAF mutation indication
Jakavi Hematology Myelofibrosis (MF),
polycytomia vera (PV),
graft-versus-host disease
(GvHD)
Zolgensma Neuroscience Spinal muscular atrophy
(SMA)
Xolair
Immunology Severe allergic asthma (SAA),
chronic spontaneous urticaria
(CSU), nasal polyps
Sandostatin Established Brands Carcinoid tumors,
acromegaly
Kisqali Solid Tumors HR+/HER-
metastatic breast cancer
Ilaris Immunology Auto-inflammatory (CAPS,
TRAPS, HIDS/MKD, FMF,
SJIA, AOSD, gout)
Kesimpta Neuroscience Relapsing-remitting
multiple sclerosis (RRMS)
Galvus Group Established Brands Type diabetes
Gleevec/Glivec Established Brands Chronic myeloid
leukemia (CML),
gastrointestinal stromal
tumors (GIST)
Exforge Group Established Brands Hypertension
Diovan Group Established Brands Hypertension
Kymriah Hematology r/r pediatric and young
adults acute lymphoblastic
leukemia (ALL), diuse large
B-cell lymphoma (DLBCL),
follicular lymphoma (FL)
Afinitor/Votubia Established Brands Breast cancer/
tuberous sclerosis complex
(TSC)
Top brands total
Rest of portfolio
Total division net
sales to third parties
1
Net sales to third parties reflect Xolair sales for all indications.

Notes to the Novartis Groupconsolidatedfinancial statements
F-24
Net sales to third parties of the top 20 Innovative Medicines Division brands in 2021
Brand classification by
therapeutic area, other
Rest of
promoted brands or US
world
Tot a l
Brands established brands
Key indications USD m
USD m
USD m
Cosentyx Immunology Psoriasis (PsO), ankylosing
spondylitis (AS),
psoriatic arthritis (PsA),
non-radiographic axial
spondyloarthritis (nr-axSPA)
Entresto Cardiovascular Chronic heart failure
Gilenya Neuroscience Relapsing multiple sclerosis (RMS)
Lucentis Other Promoted Age-related macular
Brands degeneration (AMD)
Tasigna Hematology Chronic myeloid leukemia (CML)
Promacta/Revolade Hematology Immune thrombocytopenia (ITP),
severe aplastic anemia (SAA)
Tafinlar + Mekinist Solid Tumors BRAF V+ metastatic
adjuvant melanoma,
advanced non-small cell
lung cancer (NSCLC)
Jakavi Hematology Myelofibrosis (MF),
polycythemia vera (PV)
Xolair
Immunology Severe allergic asthma (SAA),
chronic spontaneous urticaria
(CSU), nasal polyps
Sandostatin Established Brands Carcinoid tumors, acromegaly
Zolgensma Neuroscience Spinal muscular atrophy (SMA)
Galvus Group Established Brands Type diabetes
Ilaris Immunology Auto-inflammatory (CAPS,
TRAPS, HIDS/MKD, FMF,
SJIA, AOSD, gout)
Gleevec/Glivec Established Brands Chronic myeloid leukemia
(CML), gastrointestinal
stromal tumors (GIST)
Afinitor/Votubia Established Brands Breast cancer/
tuberous sclerosis complex (TSC)
Kisqali Solid Tumors HR+/HER-
metastatic breast cancer
Exforge Group Established Brands Hypertension
Diovan Group Established Brands Hypertension
Kymriah Hematology r/r pediatric and young
adults acute lymphoblastic
leukemia (ALL), diuse large
B-cell lymphoma (DLBCL)
Ultibro Group Other Promoted Cronic obstructive
Brands pulmonary disease
(COPD)
Top products total
Rest of portfolio
Total division net
sales to third parties
1
Brand classifications have been changed to reflect the new Innovative Medicines divisional structures announced on April 4, 2022.
2
Net sales to third parties reflect Xolair sales for all indications.

Notes to the Novartis Groupconsolidatedfinancial statements
F-25
Net sales to third parties of the top 20 Innovative Medicines Division brands in 2020
Brand classification by
therapeutic area, other
Rest of
promoted brands or US
world
Tot a l
Brands established brands
Key indications USD m
USD m
USD m
Cosentyx Immunology Psoriasis (PsO), ankylosing
spondylitis (AS), psoriatic arthritis (PsA)
Gilenya Neuroscience Relapsing multiple sclerosis (RMS)
Entresto Cardiovascular Chronic heart failure
Tasigna Hematology Chronic myeloid leukemia (CML)
Lucentis Other Promoted Age-related macular
Brands degeneration (AMD)
Promacta/Revolade Hematology Immune
thrombocytopenia (ITP),
severe aplastic anemia (SAA)
Tafinlar + Mekinist Solid Tumors BRAF V+ metastatic
adjuvant melanoma,
advanced non-small cell
lung cancer (NSCLC)
Sandostatin Established Brands Carcinoid tumors,
acromegaly
Jakavi Hematology Myelofibrosis (MF),
polycythemia vera (PV)
Xolair
Immunology Severe allergic asthma (SAA),
chronic spontaneous urticaria
(CSU)
Galvus Group Established Brands Type diabetes
Gleevec/Glivec Established Brands Chronic myeloid leukemia (CML),
gastrointestinal stromal tumors (GIST)
Afinitor/Votubia Established Brands Breast cancer/
tuberous sclerosis complex (TSC)
Diovan Group Established Brands Hypertension
Exforge Group Established Brands Hypertension
Zolgensma Neuroscience Spinal muscular atrophy
(SMA)
Ilaris Immunology Auto-inflammatory (CAPS,
TRAPS, HIDS/MKD, FMF,
SJIA, AOSD gout)
Kisqali Solid Tumors HR+/HER-
metastatic breast cancer
Exjade/Jadenu Established Brands Chronic iron overload
Votrient Solid Tumors Renal cell carcinoma (RCC)
Top products total
Rest of portfolio
Total division net
sales to third parties
1
Brand classifications have been changed to reflect the new Innovative Medicines divisional structures announced on April 4, 2022.
2
Net sales to third parties reflect Xolair sales for all indications.
Sandoz Division net sales to third parties by business franchise
Change
Change
( to
( to
)
)
USD m
USD m
USD
USD m
USD
Retail Generics
–
–
Biopharmaceuticals
–
Anti-Infectives
–
–
Total division net sales to third parties
–
1
Sandoz total anti-infectives net sales to third parties amounted to USD 1.2 billion (2021: USD 1.1 billion; 2020: USD 1.2 billion), of which USD 777 million (2021: USD 707 million; 2020:
USD 694 million) is sold through the Retail Generics business franchise and USD 380 million (2021: USD 423 million; 2020: USD 474 million) is sold to other third-party companies
through the Anti-Infectives business franchise.
The product portfolio of Sandoz is widely spread in 2022, 2021 and 2020.

Notes to the Novartis Groupconsolidatedfinancial statements
F-26
Segmentation – other revenue
Innovative Medicines Sandoz Corporate Group
(including eliminations)
(USD millions)
Profit-sharing income
Royalty income
Milestone income
Other
Total other revenues
1
Other includes revenue from activities such as manufacturing or other services rendered, to the extent such revenue is not recorded under net sales to third parties.
4. Associated companies
Net income statement eect Other comprehensive income eect
1
Total comprehensive income eect
(USD millions)
Roche Holding AG, Switzerland
–
Others –
–
–
–
–
–
Associated companies –
–
–
1
In 2021, Novartis share of other comprehensive income recognized by associated companies, net of taxes of USD 3 million was recycled into the consolidated income statement as
a result of the divestment of the investment in Roche Holding AG. No Novartis share of other comprehensive income recognized by associated companies was recycled to the
consolidated income statement in 2022 and 2020.
Novartis has certain non-significant investments and had
a significant investment in Roche Holding AG, Basel
(Roche), which was divested to Roche on December 6,
2021, that are accounted for as associated companies.
Roche Holding AG
On November 3, 2021, Novartis entered into an agree-
ment with Roche Holding AG to divest its 33.3% of Roche
Holding AG (Roche) voting shares, representing approx-
imately 6.2% of Roche’s total outstanding voting and
non-voting equity instruments, to Roche for USD 20.7
billion in cash. As a result, Novartis discontinued the use
of equity method accounting starting from November 3,
2021.
The divestment transaction closed on December 6,
2021, and Novartis realized a gain of USD 14.6 billion,
recorded in income from associated companies. See
Note 2.
The Group’s holding in Roche voting shares was
33.3% at December 31, 2020. This investment repre
-
sented approximately 6.2% of Roche’s total outstanding
voting and non-voting equity instruments at December
31, 2020.
Since full-year financial data for Roche is not avail-
able when Novartis produces its consolidated financial
results, a survey of analyst estimates is used to estimate
the Group’s share of Roche’s net income. Any dierences
between these estimates and actual results were
adjusted in the Group’s consolidated financial state
-
ments when available. As Novartis discontinued the use
of equity method accounting starting from November 3,
2021, and the divestment closed on December 6, 2021,
no such adjustment has been made to the 2022 Group’s
consolidated financial statements.
In 2021, dividends received from Roche in relation to
the distribution of its 2020 net income amounted to
USD522 million.
The consolidated income statement eects from
applying Novartis accounting principles for this invest-
ment in 2021 and 2020 are as follows:
(USD millions)
Novartis share of Roche’s
estimated current-year
consolidated net income
Prior-year adjustment
–
Amortization of fair value
adjustments relating to
intangible assets, net of taxes
of : USD
million; : USD million –
–
Gain on divestment of the
investment in Roche
Net income eect
1
The gain on divestment of the investment in Roche includes the recycling of currency
translation eects (see Note 8.1) and other comprehensive income eects totaling
USD 3.2 billion.
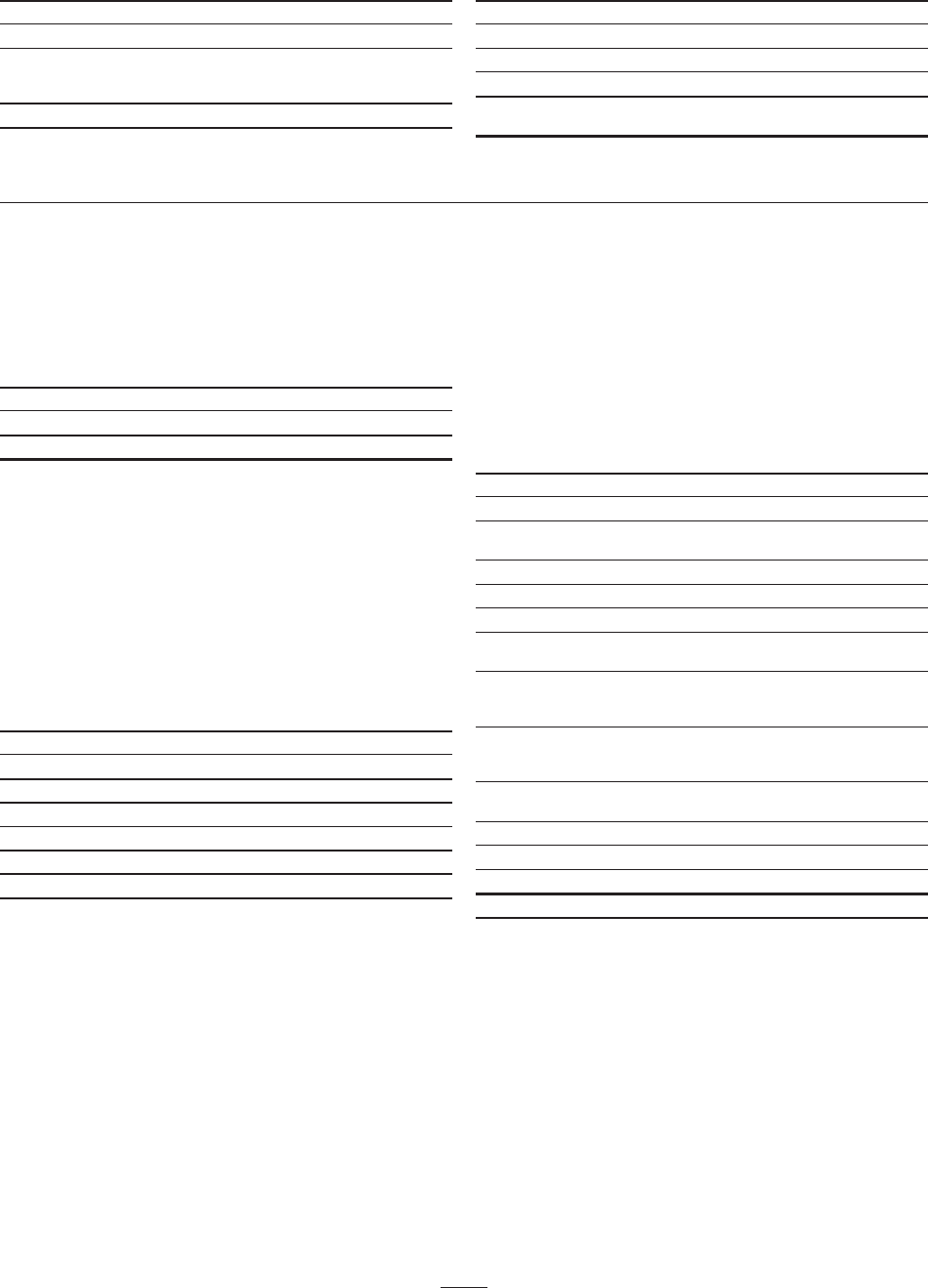
Notes to the Novartis Groupconsolidatedfinancial statements
F-27
5. Interest expense
andotherfinancialincomeandexpense
Interest expense
(USD millions)
Interest expense –
–
–
Interest expense on lease liabilities –
–
–
Expense arising from
discounting long-term liabilities
and capitalized borrowing costs –
–
–
Total interest expense –
–
–
Other financial income and expense
(USD millions)
Interest income
Other financial income
Financial expense –
–
–
Currency result, net –
–
–
Total other financial income
and expense
–
–
6. Income taxes
Income before taxes
(USD millions)
Switzerland
Foreign
Income before taxes
1
The 2021 income before taxes in Switzerland includes a USD 14.6 billion non-taxable
gain on the divestment of the Group’s investment in Roche Holding AG (see Note 2
and Note 4).
Current and deferred income tax expense
The significant components of the provision for income
taxes are as follows:
(USD millions)
Switzerland –
–
–
Foreign –
–
–
Current income tax expense –
–
–
Switzerland –
–
Foreign
Deferred tax income
Income tax expense –
–
–
Analysis of tax rate
Novartis has a substantial business presence in many
countries and is therefore subject to income taxes in dif-
ferent tax jurisdictions. This leads to dierences in
income and expense items that are non-taxable or
non-deductible (permanent dierences) or are taxed at
dierent statutory tax rates in those tax jurisdictions. As
a result, there is a dierence between our applicable tax
rate and eective tax rate.
The applicable tax rate changes from year to year
due to changes in the mix of the Group’s pre-tax income
and changes in statutory tax rates since it is calculated
as the weighted average tax rate based on the pre-tax
income of each subsidiary.
The main elements contributing to the dierence
between the Group’s overall applicable tax rate and the
eective tax rate are shown in the following table:
(As a percentage)
Applicable tax rate
Eect of disallowed expenditures
Eect of utilization of previously unrecognized
tax losses brought forward from prior periods
–
Eect of income taxed at reduced rates –
–
–
Eect of income not subject to tax
–
–
–
Eect of tax credits and allowances –
–
–
Eect of release of
contingent consideration liability –
–
–
Eect of tax rate change
on current and deferred
tax assets and liabilities –
Eect of derecognition and
reversals of derecognition
of deferred tax assets
Eect of write-down and reversal of
write-down of investments in subsidiaries
–
Eect of prior-year items –
Eect of changes in uncertain tax positions
Eect of other items
–
Eective tax rate
1
2021 includes the eect of income not subject to tax (– 7.3%) arising from the
non-taxable gain on the divestment of our investment in Roche. See Notes 2 and 4 for
further details.
Our eective tax rate fluctuates based primarily on,
among other factors, changes in pre-tax income between
countries with varying statutory tax rates, income taxed
at reduced tax rates, eect of disallowed expenditures,
eect of income not subject to tax, eect of tax credits
and allowances, eect of prior-year items, changes in
the measurement of deferred tax assets, changes in
uncertain tax positions and changes in tax laws. The
table above provides the details of the significant items

Notes to the Novartis Groupconsolidatedfinancial statements
F-28
that impact the comparability of the eective tax rate
between years.
The utilization of tax-loss carry-forwards lowered the
tax charge by USD1 million in 2022, by USD5 million in
2021, and by USD29 million in 2020.
7. Earnings per share
Net income attributable to shareholders of Novartis AG (USD millions)
Number of shares (in millions)
Weighted average number of shares outstanding used in basic earnings per share
Adjustment for vesting of restricted shares, restricted share units and dilutive shares from options
Weighted average number of shares in diluted earnings per share
Basic earnings per share (USD)
Diluted earnings per share (USD)
Basic earnings per share (EPS) is calculated by dividing
net income attributable to shareholders of Novartis AG
by the weighted average number of shares outstanding
in a reporting period. This calculation excludes the aver-
age number of issued shares purchased by the Group
and held as treasury shares.
For diluted EPS, the weighted average number of
shares outstanding is adjusted to assume the vesting of
all restricted shares, restricted share units, and the
conversion of all potentially dilutive shares arising from
options on Novartis shares that have been issued.
No options were excluded from the calculation of
diluted EPS in 2022, 2021 or 2020, as all options were
dilutive in all years.

Notes to the Novartis Groupconsolidatedfinancial statements
F-29
8. Changes in consolidated statements
ofcomprehensive income
The consolidated statements of comprehensive income
include the Group’s net income for the year as well as all
other valuation adjustments recorded in the Group’s con-
solidated balance sheet, which under IFRS are not
recorded in the consolidated income statement. These
include fair value adjustments on financial instruments,
actuarial gains or losses on defined benefit pension
plans, and currency translation eects, net of taxes.
Total value
Fair value
Actuarial
Cumulative
adjustments
adjustments
gains/(losses)
currency
attributable to
Non-
on financial
from defined
translation
Novartis AG
controlling
Total value
(USD millions) Note
instruments
benefit plans
eects
shareholders
interest
adjustments
Value adjustments at December ,
–
–
–
–
Fair value adjustments on equity securities,
net of taxes of USD - million
Net investment hedge
–
–
–
Defined benefit plans, net of taxes
of USD - million
–
Currency translation eects,
net of taxes of USD million
Total value adjustments in
–
Fair value adjustments on equity securities
sold, reclassified to retained earnings
–
–
–
Value adjustments related to divestments
Impact of change in ownership of consolidated entities
–
–
Value adjustments at December ,
–
–
–
–
Fair value adjustments on equity securities,
net of taxes of USD - million
Net investment hedge, net of taxes
of USD million
Defined benefit plans, net of taxes
of USD - million
Currency translation eects,
net of taxes of USD million
–
–
–
–
Total value adjustments in
–
–
–
–
Fair value adjustments on equity securities
sold, reclassified to retained earnings
net of taxes of USD million
–
–
–
Value adjustments related to divestments
– –
–
–
Value adjustments at December ,
–
–
–
–
–
Fair value adjustments on equity securities,
net of taxes of USD million
–
–
–
Net investment hedge, net of taxes
of USD - million
Defined benefit plans, net of taxes
of USD - million
–
–
–
Currency translation eects,
net of taxes of USD million
–
–
–
–
Total value adjustments in
–
–
–
–
–
–
Fair value adjustments on equity securities
sold, reclassified to retained earnings
net of taxes of nil
–
–
–
Value adjustments related to divestments,
net of taxes of USD - million
Value adjustments at December ,
–
–
–
–
–
–
1
Includes fair value adjustments on equity securities designated as financial assets valued at fair value through other comprehensive income with no subsequent recycling into the
consolidated income statement

Notes to the Novartis Groupconsolidatedfinancial statements
F-30
8.1) In 2022, net cumulative currency translation gains of
USD 13 million were recycled through the income state-
ment as a result of the divestments of subsidiaries. In
2021, net cumulative currency translation gains of USD
3.2 billion were recycled through the income statement
as a result of the divestment of the investment in Roche.
See Notes 2 and 4. In 2020, there were no currency
translation losses or gains recycled through the income
statement.
9. Property, plant and equipment
The following table summarizes the movements of property, plant and equipment during 2022:
Machinery
Construction
and other
(USD millions) Land
Buildings
in progress
equipment
Total
At January ,
Cost
Accumulated depreciation and impairment –
–
–
–
–
Net book value
At January ,
Impact of acquisitions of businesses
Reclassifications
–
Additions
Disposals and derecognitions –
–
–
–
–
Depreciation charge
–
–
–
Impairment charge –
–
–
–
–
Reversal of impairment charge
Currency translation eects –
–
–
–
–
At December ,
At December ,
Cost
Accumulated depreciation and impairment –
–
–
–
–
Net book value
Commitments for purchases of property, plant and equipment
Capitalized borrowing costs

Notes to the Novartis Groupconsolidatedfinancial statements
F-31
The following table summarizes the movements of property, plant and equipment during 2021:
Machinery
Construction
and other
(USD millions) Land
Buildings
in progress
equipment
Total
At January ,
Cost
Accumulated depreciation and impairment –
–
–
–
–
Net book value
At January ,
Reclassifications
–
Additions
Disposals and derecognitions –
–
–
–
–
Depreciation charge
–
–
–
Impairment charge –
–
–
–
–
Reversal of impairment charge
Currency translation eects –
–
–
–
–
At December ,
At December ,
Cost
Accumulated depreciation and impairment –
–
–
–
–
Net book value
Commitments for purchases of property, plant and equipment
Capitalized borrowing costs
The following table shows the property, plant and equipment impairment charges and reversals by reporting seg-
ment:
Impairment charges Impairment reversals
(USD millions)
Innovative Medicines –
–
–
Sandoz –
–
–
Corporate
–
Total –
–
–

Notes to the Novartis Groupconsolidatedfinancial statements
F-32
10. Right-of-use assets and lease liabilities
The following table summarizes the movements of the
right-of-use assets:
(USD millions)
Right-of-use assets at January
Impact of acquisitions of businesses
Additions
Depreciation charge –
–
Impairment charge
–
Lease contract terminations
–
–
Currency translation eects –
–
Total right-of-use assets at December
1
Impairment charges in 2022 were recorded in the Innovative Medicines segment.
2
Lease contract terminations also includes modifications to existing leases that result
in reductions to the right-of-use assets, and reductions due to sub-leasing.
The following table shows the right-of-use assets carry-
ing value and depreciation charge at December 31, 2022
and 2021, by underlying class of asset:
December ,
Depreciation
December ,
Depreciation
charge
charge
(USD millions) carrying value
carrying value
Land
Buildings
Vehicles
Machinery and
equipment, and
other assets
Total right-of-use
assets
The following table shows the lease liabilities by maturity at December 31, 2022 and 2021:
Lease liabilities
Lease liabilities
Lease liabilities
undiscounted
Lease liabilities
undiscounted
(USD millions)
Less than one year
Between one and two years
Between two and three years
Between three and four years
Between four and five years
After five years
Total lease liabilities
Less current portion of lease liabilities –
–
–
–
Non-current portion of lease liabilities
Commitments for leases not yet commenced
At December 31, 2022, and December 31, 2021, there
were no material future cash outflows, including exten-
sion options, excluded from the measurement of lease
liabilities. The Group’s most material lease with a lease
term extension, representing a lease liability value of USD
0.7 billion (2021: USD 0.6 billion), has a determined lease
term end date of 2071 (2021: 2071). Non-enforceable
extension options of up to 10 years have not been
included within the measurement of this lease liability,
and do not have a material impact to the carrying value
of the lease for both 2022 and 2021. Should the landlord
agree to a lease extension, rent will be referenced to the
market rates as at the commencement of the extension
period.
In 2022, the Group completed three sale and lease-
back transactions for certain property, plant and equip-
ment as part of the Groups plans to focus on key oper-
ating locations. The transactions resulted in net cash
inflows of USD 49 million and the recognition of USD 23
million of lease liabilities, and USD 13 million of right-of-
use assets. The right-of-use assets value reflects the
proportion of the property, plant and equipment retained.
Extension options have been included where manage-
ment believe that such options will be exercised. The lia-
bilities reflect the net present value of future lease
payments. The net gain on the sale and leaseback trans-
actions amounted to USD 17 million. There were no sig-
nificant sale and leaseback transactions in 2021 or 2020.
The following table provides additional disclosures
related to right-of-use assets and lease liabilities for
2022, 2021 and 2020:
(USD millions)
2021
2020
Interest expense on lease liabilities
Expense on short-term leases
Expense on low-value leases
Total cash outflows for leases
Thereof:
Cash outflows for short-term leases
and low-value leases
9
13
11
Payments of interest
51
52
56
Payments of lease liabilities
295
316
312
1
The weighted average interest rate is 3.3% (2021: 3.2%, 2020: 3.4%).
2
Cash flows from short-term and low-value leases are included within total net cash
flows from operating activities. The portfolio of short-term leases to which the Group
is committed to at December 31, 2022, 2021 and 2020, is similar to the portfolio of
short-term leases the Group entered into during 2022, 2021 and 2020.
3
Included within total net cash flows from operating activities
4
Reported as cash outflows in financing activities net of lease incentives received, if
any.

Notes to the Novartis Groupconsolidatedfinancial statements
F-33
The net investment held and income from subleasing
right-of-use assets were not significant for 2022, 2021,
and 2020. Income from leasing Novartis property, plant
and equipment to third parties for 2022, 2021 and 2020
was not significant.
11. Goodwill and intangible assets
The following table summarizes the movements of goodwill and intangible assets in 2022:
Goodwill Intangible assets other than goodwill
In-process
Currently
Other
research and
marketed
intangible
(USD millions) Total
development
Technologies
products
assets
Total
At January ,
Cost
Accumulated amortization and impairment –
–
–
–
–
–
Net book value
At January ,
Impact of acquisitions of businesses
Reclassifications
–
Additions
Disposals and derecognitions
–
–
–
–
–
Amortization charge
–
–
–
–
Impairment charge
–
–
–
–
–
Currency translation eects –
–
–
–
–
–
At December ,
At December ,
Cost
Accumulated amortization and impairment –
–
–
–
–
–
Net book value
1
Reclassifications between various asset categories as a result of product launches of acquired in-process research and development and completion of software development
2
Derecognition of assets that are no longer being used or developed and are not considered to have a significant disposal value or other alternative use

Notes to the Novartis Groupconsolidatedfinancial statements
F-34
The following table summarizes the movements of goodwill and intangible assets in 2021:
Goodwill Intangible assets other than goodwill
In-process
Currently
Other
research and
marketed
intangible
(USD millions) Total
development
Technologies
products
assets
Total
At January ,
Cost
Accumulated amortization and impairment –
–
–
–
–
–
Net book value
At January ,
Impact of acquisitions of businesses
Reclassifications
–
Additions
Disposals and derecognitions
–
–
–
Amortization charge
–
–
–
–
Impairment charge
–
–
–
–
–
Currency translation eects –
–
–
–
–
–
At December ,
At December ,
Cost
Accumulated amortization and impairment –
–
–
–
–
–
Net book value
1
Reclassifications between various asset categories as a result of product launches of acquired in-process research and development and completion of software development
2
Derecognition of assets that are no longer being used or developed and are not considered to have a significant disposal value or other alternative use
The following table summarizes the allocation of the net book values of goodwill and intangible assets by report-
ing segment at December 31, 2022:
Goodwill
Intangible assets other than goodwill
In-process
Currently
Other
research and
marketed
intangible
(USD millions) Total
development
Technologies
products
assets
Total
Innovative Medicines
Sandoz
Corporate
Net book value at December ,
1
The Innovative Medicines and Sandoz Divisions’ represent the grouping of cash-generating units, to which goodwill is allocated.
The following table summarizes the allocation of the net book values of goodwill and intangible assets by report-
ing segment at December 31, 2021:
Goodwill
Intangible assets other than goodwill
In-process
Currently
Other
research and
marketed
intangible
(USD millions) Total
development
Technologies
products
assets
Total
Innovative Medicines
Sandoz
Corporate
Net book value at December ,
1
The Innovative Medicines and Sandoz Divisions’ and Corporate represent the grouping of cash-generating units, to which goodwill is allocated.
As at December 31, 2022, the most significant intangi-
ble assets within currently marketed products category
are Leqvio (Innovative Medicines: acquisition of The
Medicines Company) and Zolgensma (Innovative
Medicines: acquisition of Avexis Inc.). As at December
31, 2022, the carrying value and remaining amortization
period for Leqvio is USD 7.4 billion and 13 years, respec-
tively (2021: USD 7.9 billion and 14 years, respectively),
and for Zolgensma USD 5.9 billion and 8 years, respec-
tively (2021: USD 6.6 billion and 9 years, respectively).
The Innovative Medicines and Sandoz Divisions’
cash-generating units, to which goodwill is allocated,
Notes to the Novartis Groupconsolidatedfinancial statements
F-35
each comprise a group of smaller cash-generating units.
The valuation method of the recoverable amount of the
group of cash-generating units, to which goodwill is allo-
cated, is based on the fair value less costs of disposal.
The following assumptions are used in the calcula-
tions:
Innovative
(As a percentage) Medicines
Sandoz
Terminal growth rate
Discount rate (post-tax)
The discount rates for all divisions consider the Group’s
weighted average cost of capital, adjusted to
approximate the weighted average cost of capital of a
comparable market participant.
The fair value less costs of disposal, for all cash-gen-
erating units containing goodwill, is reviewed for the
impact of reasonably possible changes in key assump-
tions. In particular, we considered an increase in the dis-
count rate, a decrease in the terminal growth rate, and
certain negative impacts on the forecasted cash flows.
These reasonably possible changes in key assumptions
did not indicate an impairment.
“Note 1. Significant accounting policies—Impairment
of goodwill and intangible assets” provides additional
disclosures on how the Group performs goodwill and
intangible asset impairment testing.
The following table shows the intangible asset impairment charges and reversals by reporting segment:
Impairment charges Impairment reversals
(USD millions)
Innovative Medicines
–
–
–
Sandoz –
–
–
Corporate –
–
–
Total –
–
–
1
2022 includes an impairment of USD 0.6 billion related to the write-down of IPR&D related to cessation of clinical development program UNR844.
2021 includes an impairment of USD 0.2 billion related to the write-down of IPR&D related to cessation of clinical development program GTX312.
2020 includes an impairment of USD 0.5 billion related to the write-down of IPR&D related to cessation of clinical development program ZPL389 for atopic dermatitis and USD 0.2
billion related to a partial write-down of the Votrient currently marketed product (Votrient carrying value was USD 0.9 billion in 2022 and USD 1.3 billion in 2021).

Notes to the Novartis Groupconsolidatedfinancial statements
F-36
12. Deferred tax assets and liabilities
Pensions and
Property,
other benefit
Tax loss
Other assets,
plant and
Intangible
obligations
carry-
provisions
(USD millions) equipment
assets
of employees
Inventories
forwards
and accruals
Total
Gross deferred tax assets at January ,
Gross deferred tax liabilities at January , –
–
–
–
–
–
Net deferred tax balance at January , –
–
At January , –
–
Credited/(charged) to income
–
–
Charged to equity
Credited/(charged) to other comprehensive income –
–
–
Impact of acquisitions of businesses
–
–
Other movements
–
–
–
Net deferred tax balance at December , –
–
Gross deferred tax assets at December ,
Gross deferred tax liabilities at December , –
–
–
–
–
–
Net deferred tax balance at December , –
–
After osetting the following amount of deferred tax
assets and liabilities within the same tax jurisdiction,
the balance amounts to:
Deferred tax assets at December ,
Deferred tax liabilities at December ,
–
Net deferred tax balance at December ,
Gross deferred tax assets at January ,
Gross deferred tax liabilities at January , –
–
–
–
–
–
–
Net deferred tax balance at January , –
–
At January , –
–
Credited/(charged) to income –
–
–
–
Charged to equity
–
–
Credited/(charged) to other comprehensive income
–
–
Impact of acquisitions of businesses
–
–
Other movements
–
–
–
–
–
Net deferred tax balance at December , –
–
Gross deferred tax assets at December ,
Gross deferred tax liabilities at December , –
–
–
–
–
–
Net deferred tax balance at December , –
–
After osetting the following amount of deferred tax
assets and liabilities within the same tax jurisdiction,
the balance amounts to:
Deferred tax assets at December ,
Deferred tax liabilities at December ,
–
Net deferred tax balance at December ,
Notes to the Novartis Groupconsolidatedfinancial statements
F-37
Deferred tax liabilities have not been recognized for the
withholding tax and other taxes that would be payable
on the remittance of earnings of foreign subsidiaries,
insofar as the Group has the ability to control any future
reversal and the unremitted earnings are retained in the
foreign subsidiaries for reinvestment. The total unremit-
ted earnings retained for reinvestment in the Group’s for-
eign subsidiaries that would be subject to withholding
tax or other taxes if remitted to the Group are estimated
at approximately USD 32 billion in 2022, (2021: USD 29
billion).
The gross value of tax-loss carry-forwards that have or
have not been recognized as deferred tax assets, with
their expiry dates, is as follows:
(USD millions) Unrecognized
Recognized
total
One year
Two years
Three years
Four years
Five years
More than five years
Not subject to expiry
Total
(USD millions) Unrecognized
Rcognized
total
One year
Two years
Three years
Four years
Five years
More than five years
Not subject to expiry
Tot al
(USD millions)
Tax losses carried forward
that expired
Deferred tax assets related to carry-forwards of taxable
losses and tax credits of relevant Group entities are rec-
ognized to the extent it is considered probable that future
taxable profits will be available in the respective tax juris-
dictions against which such losses and credits can be
utilized.
13. Financial and other non-current assets
Financial assets
(USD millions)
Equity securities
Debt securities
Fund investments
Total financial investments
Long-term receivables from finance subleases
Other long-term receivables
Contingent consideration receivables
Long-term loans, advances and security deposits
Total financial assets
1
Note 29 provides additional disclosures related to contingent consideration.
Other non-current assets
(USD millions)
Deferred compensation plans
Prepaid post-employment benefit plans
Other non-current assets
Total other non-current assets
1
Note 25 provides additional disclosures related to post-emplyment benefits.

Notes to the Novartis Groupconsolidatedfinancial statements
F-38
14. Inventories
(USD millions)
Raw material, consumables
Work in progress
Finished products
Total inventories
The following table shows the amount of inventory rec-
ognized as an expense in “Cost of goods sold” in the
consolidated income statements:
(USD billions)
Cost of goods sold –
–
–
The following table shows the recognized amount of
inventory provision and reversals of inventory provision
recorded in the consolidated income statements:
(USD millions)
Inventory provisions –
–
–
Reversals of inventory provisions
The reversals mainly result from the release of products
initially requiring additional quality control inspections
and from the reassessment of inventory values manu-
factured prior to regulatory approval but for which
approval was subsequently received.
15. Trade receivables
(USD millions)
Total gross trade receivables
Provisions for doubtful trade receivables –
–
Total trade receivables, net
The following table summarizes the movement in the provision for doubtful trade receivables:
(USD millions)
January –
–
–
Provisions for doubtful trade receivables charged to the consolidated income statement –
–
–
Utilization of provisions for doubtful trade receivables
Reversal of provisions for doubtful trade receivables credited to the consolidated income statement
Currency translation eects
–
December –
–
–
The following table shows the trade receivables that are
not overdue as specified in the payment terms and con-
ditions established with Novartis customers, as well as
an analysis of overdue amounts and related provisions
for doubtful trade receivables:
(USD millions)
Not overdue
Past due for not more than one month
Past due for more than one month
but less than three months
Past due for more than three months
but less than six months
Past due for more than six months
but less than one year
Past due for more than one year
Provisions for doubtful trade receivables –
–
Total trade receivables, net
Trade receivable balances represent amounts due from
our customers, which are mainly drug wholesalers, retail-
ers, private health systems, government agencies, man-
aged care providers, pharmacy benefit managers and
government-supported healthcare systems. We partic-
ularly monitor the level of trade receivables in countries
deemed to have an elevated credit risk. We consider
macroeconomic environment, historical experience,
country and political risk, in addition to other relevant
information when assessing risk. These risk factors are
monitored regularly to determine any adjustments in risk
classification. The majority of the past due trade receiv-
ables from elevated credit risk countries are due from
local governments or from government-funded entities.
Deteriorating credit and economic conditions as well as
other factors in these elevated credit risk countries have
resulted in, and may continue to result in, an increase in
the average length of time that it takes to collect these

Notes to the Novartis Groupconsolidatedfinancial statements
F-39
trade receivables, and may require the Group to re-eval-
uate the expected credit loss amount of these trade
receivables in future periods. At December 31, 2022,
amounts past due for more than one year are not signif-
icant in elevated credit risk countries.
Total trade receivables include amounts denomi-
nated in the following major currencies:
(USD millions)
US dollar (USD)
Euro (EUR)
Russian ruble (RUB)
Japanese yen (JPY)
British pound (GBP)
Chinese yuan (CNY)
Canadian dollar (CAD)
Brazilian real (BRL)
Australian dollar (AUD)
Swiss franc (CHF)
Other currencies
Total trade receivables, net
16. Marketable securities, commodities, timedeposits,
derivative financial instruments, andcash and cash
equivalents
Marketable securities, commodities, timedeposits and derivative financial instruments
(USD millions)
Commodities
Debt securities
Time deposits and short-term investments with original maturity more than days
Derivative financial instruments
Total marketable securities, commodities, time deposits and derivative financial instruments
The vast majority of debt securities, time deposits and short-term investments with an original maturity of more
than 90 days was denominated in USD as of December 31, 2022, and 2021.
Cash and cash equivalents
(USD millions)
Current accounts
Time deposits and short-term investments with original maturity less than days
Total cash and cash equivalents
Notes to the Novartis Groupconsolidatedfinancial statements
F-40
17. Other current assets
(USD millions)
VAT receivable
Withholding tax recoverable
Prepaid expenses
Contingent consideration receivable
Other receivables and current assets
Total other current assets
1
Note 29 provides additional disclosures related to contingent consideration.
18. Equity
The following table shows the movement in the share capital:
Movement
Movement
Movement
(USD millions) Jan ,
in year
Dec ,
in year
Dec ,
in year
Dec ,
Share capital
–
–
–
Treasury shares –
–
–
–
–
Outstanding share capital
–
–
1
The Novartis AG share capital consists of registered shares with a nominal value of CHF 0.50 each. No authorized and conditional capital exists.
The following table shows the movement in the shares:
2022 2021 2020
Total
Total
Total
Total
Total
Total
Total
Total
Total
Number of outstanding shares
Novartis
treasury
outstanding
Novartis
treasury
outstanding
Novartis
treasury
outstanding
(in millions) Note
shares
shares
shares
shares
shares
shares
shares
shares
shares
Balance at beginning of year
–
–
–
Shares canceled for capital
reduction
–
–
–
Shares acquired to be
canceled
–
–
–
–
–
–
Other share purchases
–
–
–
–
–
–
Exercise of options
and employee transactions
Equity-based compensation
Shares delivered to Alcon
employees
Total movements
–
–
–
–
–
–
–
Balance at end of year
–
–
–
1
Approximately 99.0 million treasury shares (2021: 102.5 million; 2020: 103.0 million) are held in Novartis entities that restrict their availability for use.
2
Novartis reduced its share capital by canceling shares that were repurchased on the SIX Swiss Exchange second trading line during previous years.
3
Shares repurchased on the SIX Swiss Exchange second trading line under a CHF 10 billion share buyback authority approved at the 2019 Annual General Meeting (AGM) for
transactions after February 28, 2019, until March 2, 2021. Transactions after March 2, 2021, were executed under the CHF 10 billion share buyback authority approved at the 2021
AGM and the additional CHF 10 billion authority approved at the 2022 AGM.
4
Shares acquired from employees, which were previously granted to them under the respective equity-based participation plans
5
Shares delivered as a result of options being exercised and physical share deliveries related to equity-based participation plans
18.1) The amount available for distribution as a dividend
to shareholders is based on the available distributable
retained earnings of Novartis AG determined in accor-
dance with the legal provisions of the Swiss Code of
Obligations.
Dividend per share (in CHF)
Total dividend payment
(in USD billion)

Notes to the Novartis Groupconsolidatedfinancial statements
F-41
18.2) The following table summarizes the treasury shares movements:
2022 2021 2020
Number of
Number of
Number of
outstanding
outstanding
outstanding
shares
Equity impact
shares
Equity impact
shares
Equity impact
Note
(in millions)
USD m
(in millions)
USD m
(in millions)
USD m
Shares acquired to be canceled
–
–
–
–
–
–
Other share purchases
–
–
–
–
–
–
Purchase of treasury shares
–
–
–
–
–
–
Exercise of options and employee transactions
Equity-based compensation
Shares delivered to Alcon employees
Tot al
–
–
–
–
–
–
1
Shares repurchased on the SIX Swiss Exchange second trading line under a CHF 10 billion share buyback authority approved at the 2019 Annual General Meeting (AGM) for
transactions after February 28, 2019, until March 2, 2021. Transactions after March 2, 2021, were executed under the CHF 10 billion share buyback authority approved at the 2021
AGM and the additional CHF 10 billion authority approved at the 2022 AGM.
2
Shares acquired from employees, which were previously granted to them under the respective equity-based participation plans
3
Shares delivered as a result of options being exercised related to equity-based participation plans and the delivery of treasury shares. The average share price of the shares
delivered was significantly below market price, reflecting the strike price of the options exercised.
4
Equity-settled share-based compensation is expensed in the consolidated income statement in accordance with the vesting period of the share-based compensation plans. The
value for the shares and options granted is credited to consolidated equity over the respective vesting period. In addition, tax benefits arising from tax-deductible amounts
exceeding the expense recognized in the income statement are credited to equity.
18.3) In December 2021, Novartis entered into an irrevo-
cable, non-discretionary arrangement with a bank to
repurchase Novartis shares on the second trading line
under its up-to USD 15.0 billion share buyback. The
arrangement was updated in July 2022. Novartis is able
to cancel this arrangement at any time but could be sub-
ject to a 90-day waiting period.
As of December 31, 2022, these waiting period con-
ditions were not applicable and as a result, there was no
requirement to record a liability under this arrangement
as of December 31, 2022. The liability under this arrange-
ment amounted to USD 2.8 billion as of December 31,
2021.
In June 2021, Novartis entered into an irrevocable,
non-discretionary arrangement with a bank to repur-
chase Novartis shares to mitigate dilution related to par-
ticipation plans of employees. Novartis would have been
able to cancel this arrangement at any time but would
have been subject to a 90-day waiting period.
This trading plan commitment was fully executed and
expired in June 2021, and as a consequence, there is no
liability related to this plan recognized as of December
31, 2021.
In November 2020, Novartis entered into an irrevo-
cable, non-discretionary arrangement with a bank to
repurchase Novartis shares on the second trading line
under its up-to USD 2.5 billion share buyback. Novartis
would have been able to cancel this arrangement at any
time, but would have been subject to a 90-day waiting
period. The commitment under this arrangement there-
fore reflected the obligated purchases by the bank under
such trading plan over a rolling 90-day period, or if
shorter, until the maturity date of such trading plan.
The commitment under this arrangement amounted
to USD 1.8 billion as of December 31, 2020. This trading
plan commitment was fully executed and expired in
March 2021, and as a consequence, there is no liability
related to this plan recognized as of December 31, 2021.
In August 2020, Novartis entered into an irrevocable,
non-discretionary arrangement with a bank to
repurchase Novartis shares to mitigate dilution related
to participation plans of associates. Novartis would have
been able to cancel this arrangement at any time but
would have been subjected to a 90-day waiting period.
This trading plan commitment was fully executed and
expired, and as a consequence, there is no liability
related to this plan recognized as of December 31, 2020.
18.4) In October 2020, Novartis entered into an agree-
ment with the market maker for its employee options to
repurchase a portion of the outstanding written call
options. A total of 3.7 million options were repurchased
under this agreement. This agreement was terminated
in November 2020.
18.5) The impact of change in ownership of consolidated
entities represents the excess of the amount paid to
non-controlling interest over their carrying value and
equity allocation to non-controlling interest due to
change in ownership percentage.
18.6) Changes in non-controlling interests represent the
impact on the non-controlling interest of transactions
with minority shareholders, such as change in ownership
percentage, dividend payments and other equity trans-
actions.
18.7) Other movements include, for subsidiaries in hyper-
inflationary economies, the impact of the restatement of
the equity balances of the current year as well as restate-
ment of the non-monetary assets and liabilities with the
general price index at the beginning of the period. See
Note 29 for additional disclosures.
18.8) In 2021, transaction costs that were directly attrib-
utable to the distribution (spin-o) of Alcon Inc. to
Novartis AG shareholders and that would otherwise have
been avoided, were recorded to equity.

Notes to the Novartis Groupconsolidatedfinancial statements
F-42
18.9) At December 31, 2022, the market maker held 3
million (2021: 3 million; 2020: 1 million) written call options,
originally issued as part of the share-based compensa-
tion for employees, that have not yet been exercised. The
weighted average exercise price of these options is USD
66.07 (2021: USD 61.45; 2020: USD 60.09), and they
have contractual lives of 10 years, with remaining lives
less than one year (2021: two years; 2020: three years).
19. Non-current financial debt
(USD millions)
Straight bonds
Liabilities to banks and other financial institutions
Total, including current portion of non-current financial debt
Less current portion of non-current financial debt –
–
Total non-current financial debt
1
Average interest rate 2.3% (2021: 0.9%)
All bonds are initially recorded at the amount of proceeds
received, net of transaction costs. They are subsequently
carried at amortized cost, with the dierence between
the proceeds, net of transaction costs, and the amount
due on redemption being recognized as a charge to the
consolidated income statement over the period of the
relevant bond. Financial debts, including current finan-
cial debts, contain only general default covenants. The
Group is in compliance with these covenants.
The percentage of fixed-rate financial debt to total
financial debt was 86% at December 31, 2022, and 87%
at December 31, 2021.
The average interest rate on total financial debt in
2022 was 2.4% (2021: 1.9%).
Note 29 contains a maturity table of the Group’s
future contractual interest payments commitments.

Notes to the Novartis Groupconsolidatedfinancial statements
F-43
The following table provides a breakdown of straight bonds:
Notional
amount
Issuance
Maturity
(USD
(USD
Coupon Currency (millions)
year
year
Issuer Issue price
millions)
millions)
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
CHF
Novartis AG, Basel, Switzerland
CHF
Novartis AG, Basel, Switzerland
CHF
Novartis AG, Basel, Switzerland
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
USD
Novartis Capital Corporation, New York, United States
EUR
Novartis Finance S.A., Luxembourg, Luxembourg
Total straight bonds
1
The EUR 1 850 million bond issued in 2020 features a coupon step-up of 0.25% commencing with the first interest payment date after December 31, 2025, if one or both of the
2025 Patient Access Targets are not met. These 2025 Patient Access Targets are the 2025 Flagship Programs Patient Reach Target and the 2025 Strategic Innovative Therapies
Patient Reach Target, as defined in the bond prospectus. As of December 31, 2022, there is no indication that these 2025 Patient Access Targets will not be met.
The following tables provide a breakdown of total non-current financial debt, including current portion by maturity
and currency:
Breakdown by maturity:
(USD millions)
After
Total
Breakdown by currency:
(USD millions)
US dollar (USD)
Euro (EUR)
Japanese yen (JPY)
Swiss franc (CHF)
Others
Total
The following table shows the comparison of balance
sheet carrying value and fair value of total non-current
financial debt, including current portion:
Balance
Fair
Balance
Fair
(USD millions) sheet
values
sheet
values
Straight bonds
Others
Total
The fair values of straight bonds are determined by
quoted market prices. Other financial debts are recorded
at notional amounts, which are a reasonable approxima-
tion of the fair values.

Notes to the Novartis Groupconsolidatedfinancial statements
F-44
20. Provisions and other non-current liabilities
(USD millions)
Accrued liability for employee benefits:
Defined benefit pension plans
Other long-term employee benefits and deferred compensation
Other post-employment benefits
Environmental remediation provisions
Provisions for product liabilities, governmental investigations and other legal matters
Contingent consideration
Other non-current liabilities
Total provisions and other non-current liabilities
1
Note 25 provides additional disclosures related to post-employment benefits.
2
Note 29 provides additional disclosures related to contingent consideration.
Novartis believes that its total provisions are adequate
based upon currently available information. However,
given the inherent diculties in estimating liabilities in
this area, Novartis may incur additional costs beyond the
amounts provided. Management believes that such addi-
tional amounts, if any, would not be material to the
Group’s financial condition but could be material to the
results of operations or cash flows in a given period.
Environmental remediation
provisions
The following table shows the movements in the envi-
ronmental liability provisions:
(USD millions)
January
Cash payments –
–
–
Releases –
–
–
Additions
Currency translation eects –
–
December
Less current provision –
–
–
Non-current environmental
remediation provisions
at December
The significant components of the environmental reme-
diation provisions consist of costs to suciently clean
and refurbish contaminated sites to the extent neces-
sary, and to continue surveillance at sites where the envi-
ronmental remediation exposure is less significant.
A substantial portion of the environmental remedia-
tion provisions relate to the remediation of Basel regional
landfills in the adjacent border areas in Switzerland, Ger-
many and France. The provisions are reassessed on an
annual basis and adjusted as necessary.
In the United States, Novartis has been named under
federal legislation (the Comprehensive Environmental
Response, Compensation and Liability Act of 1980, as
amended) as a potentially responsible party (PRP) in
respect of certain sites. Novartis actively participates in,
or monitors, the cleanup activities at the sites in which it
is a PRP. The provision takes into consideration the
number of other PRPs at each site as well as the iden-
tity and financial position of such parties in light of the
joint and several nature of the liability.
The expected timing of the related cash outflows as
of December 31, 2022, is currently projected as follows:
Expected
(USD millions) cash outflows
Due within two years
Due later than two years, but within five years
Due later than five years, but within years
Due after years
Total environmental remediation provisions
Provisions for product liabilities,
governmental investigations and
other legal matters
Novartis has established provisions for certain product
liabilities, governmental investigations and other legal
matters where a potential cash outflow is probable and
Novartis can make a reliable estimate of the amount of
the outflow. These provisions represent the Group’s cur-
rent best estimate of the total financial eect for the mat-
ters described below and for other less significant mat-
ters. Potential cash outflows reflected in a provision
might be fully or partially oset by insurance in certain
circumstances.
Novartis has not established provisions for potential
damage awards for certain additional legal claims against
its subsidiaries if Novartis currently believes that a pay-
ment is either not probable or cannot be reliably esti-
mated. These not-provisioned-for matters include indi-
vidual product liability cases and certain other legal
matters. Plaintis’ have alleged claims in these matters
and the Group does not believe that information about
the amount sought by plaintis, if that is known, would
be meaningful with respect to those legal proceedings.
This is due to a number of factors, including, but not lim-
ited to, the stage of proceedings, the entitlement of par-
ties to appeal a decision and clarity as to theories of lia-
bility, damages and governing law. Therefore, it is not
practicable to provide information about the potential
financial impact of these matters. In addition, in some of

Notes to the Novartis Groupconsolidatedfinancial statements
F-45
these matters there are claims for punitive or multiple
(treble) damages, civil penalties and disgorgement of
profits that in the view of Novartis are either wholly or
partially unspecified, or wholly or partially unquantifiable
at present; the Group believes that information about
these amounts claimed by plaintis generally is not
meaningful for purposes of determining a reliable esti-
mate of a loss that is probable or more than remote.
A number of other legal matters are in such early
stages or the issues presented are such that the Group
has not made any provisions since it cannot currently
estimate either a potential outcome or the amount of any
potential losses. For these reasons, among others, the
Group generally is unable to make a reliable estimate of
possible loss with respect to such cases. It is therefore
not practicable to provide information about the poten-
tial financial impact of those cases.
There might also be cases for which the Group was
able to make a reliable estimate of the possible loss or
the range of possible loss, but the Group believes that
publication of such information on a case-by-case basis
would seriously prejudice the Group’s position in ongo-
ing legal proceedings or in any related settlement dis-
cussions. Accordingly, in such cases, information has
been disclosed with respect to the nature of the contin-
gency, but no disclosure is provided as to an estimate of
the possible loss or range of possible loss.
Note 28 contains additional information on contin-
gent liabilities.
Summary of significant legal
proceedings
The following is a summary of significant legal proceed-
ings to which Novartis or its subsidiaries are currently a
party, or were a party and that concluded in 2022.
Investigations and related litigations
Southern District of New York (S.D.N.Y.) Gilenya
marketing practices investigation and litigation
In 2013, Novartis Pharmaceuticals Corporation (NPC)
received a civil investigative demand from the United
States Attorney’s Oce (USAO) for the S.D.N.Y. request-
ing the production of documents and information relat-
ing to marketing practices for Gilenya, including the
remuneration of healthcare providers in connection
therewith. In 2017, the S.D.N.Y. and New York State
declined to intervene in claims raised by an individual
relator in a qui tam complaint. In 2022, NPC’s motion to
dismiss this complaint was granted, which was appealed.
The claims are being vigorously contested.
Government generic pricing antitrust investigations,
antitrust class actions
Since 2016, Sandoz Inc. has received a grand jury sub-
poena and a civil investigative demand and interrogato-
ries from the Antitrust and Civil Divisions of the US
Department of Justice (DOJ) into alleged price fixing and
market allocation of generic drugs in the United States
as well as alleged federal False Claims Act (FCA) viola-
tions. Sandoz Inc. also received a subpoena and inter-
rogatories from the Attorney General of the State of Con-
necticut in connection with a similar States’ investigation.
In 2020, Sandoz Inc. reached a resolution with the DOJ
Antitrust Division, pursuant to which Sandoz Inc. paid
USD 195 million and entered into a deferred prosecution
agreement. The Sandoz Inc. resolution related to
instances of misconduct at the Company between 2013
and 2015 with regard to certain generic drugs sold in the
United States. Under the terms of that agreement,
Sandoz Inc. will continue to take steps to enhance its
compliance program, employee training and monitoring,
and will continue to cooperate with the US government’s
ongoing investigation into the generic pharmaceutical
industry. Sandoz Inc. also finalized a resolution with the
DOJ Civil Division and in 2021 paid USD 185 million, which
includes interest from the date of the agreement in prin-
ciple, to settle related claims arising under the FCA, and
entered into a corporate integrity agreement with the
Oce of Inspector General (OIG) of the US Department
of Health and Human Services (HHS). This resolution
with the DOJ resolves all federal government matters
related to price fixing allegations.
Since the third quarter of 2016, Sandoz Inc. and Foug-
era Pharmaceuticals Inc. have been sued alongside other
generic pharmaceutical companies in numerous individ-
ual and putative class action complaints by direct and
indirect private purchasers and by over 50 US states and
territories, represented by their respective Attorneys
General. Plaintis claim that defendants, including
Sandoz Inc., engaged in price fixing and market alloca-
tion of generic drugs in the United States, and seek dam-
ages and injunctive relief. The litigation includes com-
plaints alleging product-specific conspiracies, as well as
complaints alleging the existence of an overarching
industry conspiracy, and assert claims for damages and
penalties under federal and state antitrust and consumer
protection acts. The cases have been consolidated for
pretrial purposes in the United States District Court
(USDC) for the Eastern District of Pennsylvania, and the
claims are being vigorously contested.
Lucentis/Avastin
®
matters
In connection with an investigation into whether Novartis
entities, F. Homann-La Roche AG, Genentech Inc. and
Roche S.p.A. colluded to artificially preserve the market
positions of Avastin
®
and Lucentis, in 2014 the Italian
Competition Authority (ICA) imposed a fine equivalent
to USD 125 million on the Novartis entities. Novartis paid
the fine, subject to the right to later claim recoupment,
and appealed before the Consiglio di Stato (CdS). In 2014
and 2015, the Italian Ministry of Health and the Lombar-
dia region sent letters with payment requests for a total
equivalent of approximately USD1.3 billion in damages
from Novartis and Roche entities based on these allega-
tions. In 2019, the CdS upheld the ICA decision and fine.
Following that CdS decision, several additional Italian
regions and hospitals sent letters claiming damages for
an aggregate amount of approximately USD 330 million.
None of these claims have been asserted in legal pro-
ceedings and no further letters have been sent since.
Novartis continues to appeal the CdS decision. In 2019,
the French Competition Authority (FCA) issued a State-
ment of Objections against Novartis entities, alleging
anti-competitive practices on the French market for
anti-vascular endothelial growth factor treatments for
wet age-related macular degeneration from 2008 to

Notes to the Novartis Groupconsolidatedfinancial statements
F-46
2013. In 2020, the FCA issued a decision finding that the
Novartis entities had infringed competition law by abus-
ing a dominant position and imposing a fine equivalent
to approximately USD 452 million. Novartis paid the fine,
again subject to recoupment, and is appealing the FCA’s
decision. Novartis is the subject of similar investigations
and proceedings involving competition authorities in Bel-
gium and Greece and is currently in the appeal process
in Turkey. Novartis continues to vigorously contest all
claims in all those countries. Novartis is also challeng-
ing policies and regulations allowing o-label/unlicensed
use and reimbursement for economic reasons in Turkey.
Greece investigation
The Greek authorities are investigating legacy allega-
tions of potentially inappropriate economic benefits to
HCPs, government ocials and others in Greece. These
authorities include the Greek Coordinating Body for
Inspection and Control, and the Greek Body of Prose-
cution of Financial Crime (SDOE), from which the Com-
pany received a summons in 2018 and 2020. Novartis
has cooperated in these investigations. In 2021, SDOE
imposed on Novartis Hellas a fine equivalent to approx-
imately USD 1.2 million, which Novartis Hellas has
appealed. In 2022, the Greek State served a civil lawsuit
on Novartis Hellas, seeking approximately USD 225 mil-
lion for moral damages allegedly arising from the con-
duct that was the subject of the Company’s 2020 set-
tlement with the DOJ regarding allegations of
inappropriate economic benefits in Greece that was dis-
closed in the 2020 Annual Report and the 2020 Form
20-F. The claims are being vigorously contested.
340B Drug Pricing Program investigations
In 2021, NPC received a notification from the US Health
Resources and Services Administration (HRSA) which
stated that HRSA believes NPC’s contract pharmacy pol-
icy violates the 340B statute, and threatened potential
enforcement action. NPC subsequently sued HRSA in
the USDC for the District of Columbia to challenge
HRSA’s determination and to enjoin HRSA from taking
action with respect to NPC’s contract pharmacy policy.
HRSA then referred the matter regarding NPC’s contract
pharmacy policy to OIG, which could result in the impo-
sition of civil monetary penalties on NPC. The USDC
issued a decision rejecting HRSA’s interpretation of the
340B statute, vacating the violation notification and
remanding the matter to HRSA. HRSA appealed, and the
United States Court of Appeals for the DC Circuit heard
argument on the case in 2022. In addition, in 2021, Emory
University Hospital Midtown filed an Administrative Dis-
pute Resolution (ADR) proceeding against NPC, seek-
ing the return of alleged overcharges resulting from
NPC’s contract pharmacy policy. NPC has moved to dis-
miss the proceeding pending resolution of the HRSA lit-
igation. Finally, also in 2021, NPC received a civil inves-
tigative subpoena from the Oce of the Attorney General
of the State of Vermont requesting the production of
documents and information concerning NPC’s participa-
tion in the 340B Drug Pricing Program in Vermont; NPC
provided documents and information to the Oce of the
Attorney General.
Swiss and EU investigation
In September 2022, the Swiss Competition Commission
(COMCO) initiated an investigation of Novartis acquisi-
tion of certain patents from Genentech in April 2020 and
their subsequent enforcement against Eli Lilly and other
parties, allegedly in an attempt to protect Cosentyx from
competing products. COMCO is investigating whether
enforcement of the patents violates the Swiss Cartel Act.
The European Commission also requested information
from Novartis regarding this matter. Novartis is cooper-
ating with the authorities and will vigorously contest any
allegations.
Antitrust class actions
Exforge
Since 2018, Novartis Group companies as well as other
pharmaceutical companies have been sued by various
direct and indirect purchasers of Exforge in multiple US
individual and putative class action complaints. They
claim that Novartis made a reverse payment in the form
of an agreement not to launch an authorized generic,
alleging violations of federal antitrust law and state anti-
trust, consumer protection and common laws, and seek-
ing damages as well as injunctive relief. The cases have
been consolidated in the S.D.N.Y. In 2022, Novartis
agreed to a settlement in principle to pay USD 245 mil-
lion to resolve these cases. These settlements are sub-
ject to mutually agreeable terms, finalization of docu-
mentation and, in some cases, court approval.
Product liability litigation
Reclast
NPC is a defendant in more than 20 US product liability
actions involving Reclast and alleging atypical femur
fracture injuries, all of which are in New Jersey state or
federal court and in California state court, coordinated
with claims against other bisphosphonate manufactur-
ers. The claims are being vigorously contested.
Taxotere
®
(docetaxel)
Sandoz is a defendant in more than 3 100 US product
liability actions involving Taxotere
®
(docetaxel), an
oncology product, many of which have been transferred
to a multidistrict litigation in the Eastern District of Lou-
isiana. The complaints allege misleading marketing and
that Sanofi, as innovator, and several 505(b)(2) NDA hold-
ers (including Sandoz) failed to warn of the risk of per-
manent alopecia/hair loss. In 2022, actions involving
claims related to alleged eye injuries caused by the use
of Ta xotere
®
were coordinated in a separate multidistrict
litigation in the Eastern District of Louisiana. The claims
are being vigorously contested.
Amiodarone
Sandoz entities are named in two multi-plainti US prod-
uct liability cases involving amiodarone, a cardiac drug
indicated to treat life-threatening arrhythmias that have
not responded to other treatment. The complaints allege
failure to warn, o-label promotion, and failure to include
medication guides to pharmacies. The claims are being
vigorously contested.

Notes to the Novartis Groupconsolidatedfinancial statements
F-47
Sartans and ranitidine
Since 2018, claims have been brought against Sandoz
and other pharmaceutical companies alleging injury from
carcinogenic impurities found in valsartan and valsartan/
HCT film-coated tablets and/or losartan marketed or
manufactured by Sandoz. These claims include several
putative class actions in Canada. Claims have also been
brought alleging injury from carcinogenic impurities in
ranitidine-containing medicines. These claims also
include several putative class actions in Canada and a
multidistrict litigation in Florida. All of these claims are
being vigorously contested.
Tasigna
NPC is a defendant in more than 400 US product liabil-
ity actions involving Tasigna, alleging that the product
caused various cardiovascular eects and that NPC
failed to provide adequate warnings about those alleged
side eects. State court actions are pending in a multi-
county litigation in Bergen County, New Jersey, and fed-
eral cases are pending in a multidistrict litigation in the
Middle District of Florida. The claims are being vigorously
contested.
Other matters
Shareholder derivative lawsuit
In 2021, NPC, Sandoz Inc., Novartis Capital Corporation
and certain present and former directors and ocers of
Novartis were named as defendants, and Novartis was
named as a nominal defendant, in a purported share
-
holder derivative lawsuit filed in New York state court.
The plaintis, derivatively as purported Novartis share-
holders on behalf of Novartis, seek damages and other
remedies based on alleged conduct by the corporate
and individual defendants. In 2022, the court granted
Novartis motion to dismiss the lawsuit, which the plain-
tis have appealed.
Concluded legal matters
Average Wholesale Price (AWP) litigation –
Concluded matter
Lawsuits were brought, the latest in February 2016, by
various US state governmental entities and private par-
ties against various pharmaceutical companies, includ-
ing NPC, alleging that they fraudulently overstated the
AWP that is or has been used by payers, including state
Medicaid agencies, to calculate reimbursements to
healthcare providers. In 2022, NPC settled a putative
class action brought by private payers in New Jersey,
which resolved the last AWP lawsuit. This matter is now
concluded.
Entresto matter– Concluded matter
In 2021, NPC received a civil investigative demand from
the DOJ seeking information from 2016 to the present
regarding the marketing and pricing of Entresto, includ-
ing remuneration provided to HCPs. In December 2022,
the DOJ advised that it has no additional requests and
that the matter is considered closed. This matter is now
concluded.
South Korea investigation – Concluded matter
In 2016, the Seoul Western District Prosecutor initiated
a criminal investigation into, among other things, allega-
tions that Novartis Korea utilized medical journals to pro-
vide inappropriate economic benefits to healthcare pro-
fessionals (HCPs). This resulted in a non-material fine,
which the prosecutor appealed. In 2021, the appellate
court upheld the fine, and the prosecutor appealed that
decision. In January 2023, the Supreme Court dismissed
the appeal. This matter is now concluded.
Summary of product liability, governmental
investigations and other legal matters provision
movements
(USD millions)
January
Impact of acquisitions of businesses
Cash payments –
–
–
Releases of provisions –
–
–
Additions to provisions
Currency translation eects –
–
–
December
Less current portion –
–
–
Non-current product
liabilities, governmental
investigations and other
legal matters provisions
at December
Novartis believes that its total provisions for investiga-
tions, product liability, arbitration and other legal matters
are adequate based upon currently available information.
However, given the inherent diculties in estimating lia-
bilities, there can be no assurance that additional liabil-
ities and costs will not be incurred beyond the amounts
provided.

Notes to the Novartis Groupconsolidatedfinancial statements
F-48
21. Current financial debt
andderivativefinancialinstruments
(USD millions)
Interest-bearing accounts of employees
payable on demand
Bank and other financial debt
Commercial paper
Current portion of non-current financial debt
Derivative financial instruments
Total current financial debt and derivative
financial instruments
1
Weighted average interest rate 0.25% through September 30, 2022 (2021: 0.25%)
2
Weighted average interest rate 9.7% (2021: 6.1%)
During the third quarter of 2022, Novartis closed the
interest-bearing accounts of employees payable on
demand, and paid out USD 0.9 billion to the respective
beneficiaries on October 3, 2022. The net cash outflows
from interest-bearing accounts of employees payable on
demand were reported within the line change in current
financial debts in the consolidated statements of cash
flows. See Note 23.6.
The carrying amounts of current financial debt, other
than the current portion of non- current financial debt,
approximate the estimated fair value due to the short-
term nature of these instruments.
Details on commercial papers and short-term bor-
rowings are provided under “Liquidity risk” in Note29.
22. Provisions and other current liabilities
(USD millions)
Taxes other than income taxes
Restructuring provisions
Accrued expenses for goods and services received but not invoiced
Accruals for royalties
Accrued interests on financial debt
Provisions for deductions from revenue
Accruals for compensation and benefits, including social security
Environmental remediation provisions
Deferred income
Provisions for product liabilities, governmental investigations and other legal matters
Accrued share-based payments
Contingent consideration
Commitment for repurchase of own shares
Other payables
Total provisions and other current liabilities
1
Note 20 provides additional disclosures related to legal provisions.
2
Note 29 provides additional disclosures related to contingent consideration.
3
Note 18.3 provides additional disclosures related to commitment for repurchase of own shares.
Provisions are based upon management’s best estimate and adjusted for actual experience. Such adjustments to
historic estimates have not been material.

Notes to the Novartis Groupconsolidatedfinancial statements
F-49
Provisions for deductions from revenue
The following table shows the movement of the provisions for deductions from revenue:
(USD millions)
January
Eect of currency translation, business combinations –
–
Payments/utilizations –
–
–
Adjustments of prior years charged to income statement –
–
–
Current year income statement charge
Change in provisions oset against gross trade receivables –
December
The provisions for deductions from revenue include specific healthcare plans and program rebates as well as
non-healthcare plans and program-related rebates, returns and other deductions. The provisions for deductions
from revenue are adjusted to reflect experience and to reflect actual amounts as rebates, refunds, discounts and
returns are processed. The provision represents estimates of the related obligations, requiring the use of judgment
when estimating the eect of these deductions from revenue.
Restructuring provisions movements
(USD millions)
January
Additions
Cash payments –
–
–
Releases –
–
–
Transfers –
–
Currency translation eects –
–
December
In 2022, additions to provisions of USD1.4 billion were
mainly related to the following reorganizations:
• Initiative announced in April 2022 to implement a new
streamlined organizational model designed to support
innovation, growth and productivity.
• The continuation of the Innovative Medicines Division
and the Operation unit (formerly Novartis Technical
Operations and the Customer & Technology Solutions)
2021 restructuring initiatives.
In 2021, additions to provisions of USD 328 million were
mainly related to the following reorganizations:
• The Innovative Medicines Division commenced a plan
to restructure its field force and supporting functions
in response to changes in its go-to-market structure
with increased utilization of digital technology.
• Group-wide initiatives to streamline manufacturing
platforms and manufacturing functions and implement
new technologies continued. In addition, the Opera-
tions unit (formerly Customer & Technology Solutions)
continued the phased implementation of the new oper-
ating model to transition activities to service centers.
In 2020, additions to provisions of USD354 million were
mainly related to the following reorganizations:
• The Innovative Medicines Division restructured its field
force and supporting functions in Region Europe.
• The Sandoz Division initiatives to realign its organiza-
tional structures to improve competitiveness that com-
menced in 2019 continued.
• Group-wide initiatives to streamline manufacturing
platforms and manufacturing functions through the
setup of operations centers and implementation of new
technologies, in the Innovative Medicines Division and
the Sandoz Division, continued. In addition, the Oper-
ations unit (formerly Customer & Technology Solutions)
continued the phased implementation of the new oper-
ating model to change outsourcing structures and tran-
sition activities to service centers.
Notes to the Novartis Groupconsolidatedfinancial statements
F-50
23. Details to the consolidated statements of cash flows
23.1) Non-cash items and other adjustments
The following table shows the reversal of non-cash items and other adjustments in the consolidated statements of
cash flows.
(USD millions)
Depreciation, amortization and impairments on:
Property, plant and equipment
Right-of-use assets
Intangible assets
Financial assets
–
–
Change in provisions and other non-current liabilities
Gains on disposal and other adjustments on property, plant and equipment; intangible assets;
financial assets; and other non-current assets, net –
–
–
Equity-settled compensation expense
Loss/(income) from associated companies
–
–
Income taxes
Net financial expense
Other –
Total
–
1
Includes fair value changes
2
2021 included the gain of USD 14.6 billion recognized from the divestment of the Group’s investment in Roche (see Notes 2 and 4).
In 2022, other than through business combinations, there
were USD 635 million additions to intangible assets with
deferred payments. In 2022, there were USD 247 million
(2021: USD 321 million, 2020: USD 346 million) additions
to right-of-use assets recognized.
23.2) Total amount of income taxes paid
In 2022, the total amount of income taxes paid was USD2.0 billion (2021: USD2.3 billion), which was included within
“Net cash flows from operating activities.”
In 2020, the total amount of income taxes paid was USD1.9 billion, of which USD1.8 billion was included within
“Net cash flows from operating activities,” and USD88 million was included within “Net cash flows used in invest-
ing activities from discontinued operations.”
23.3) Cash flows from changes in working capital and other operating items included in
the net cash flows from operating activities
(USD millions)
(Increase)/decrease in inventories –
–
(Increase)/decrease in trade receivables –
–
Decrease in trade payables –
–
–
Change in other current and non-current assets –
–
Change in other current liabilities
Other adjustments, net
–
Total –
–
23.4) Cash flows arising from acquisitions and divestments of interests in associated
companies, net
In 2021, acquisitions and divestments of interests in associated companies, net included USD 20.7 billion proceeds
from the divestment of the Group’s investment in Roche (see Notes 2 and 4).

Notes to the Novartis Groupconsolidatedfinancial statements
F-51
23.5) Cash flows arising from acquisitions and divestments of businesses, net
The following table is a summary of the cash flow impact of acquisitions and divestments of businesses. The most
significant trans actions are described in Note 2.
(USD millions) Note
Net assets recognized as a result of acquisitions of businesses
–
–
–
Fair value of previously held equity interests
Contingent consideration payables, net
Payments, deferred consideration and other adjustments, net
–
Cash flows used for acquisitions of businesses
–
–
–
Cash flows (used for)/from divestments of businesses, net
–
Cash flows used for acquisitions and divestments of businesses, net
–
–
–
1
In 2022, USD 15 million net cash outflows from divestments of businesses included USD 20 million reduction to cash and cash equivalents due to the derecognized cash and cash
equivalents following a loss of control of a company upon expiry of an option to purchase the company, partly oset by USD 5 million net cash inflows from business divestments in
2022 and in prior years.
In 2022, the net identifiable assets of divested businesses amounted to USD 173 million, comprised of non-current assets of USD 132 million, current assets of USD 113 million,
including USD 71 million cash and cash equivalents and of non-current and current liabilities of USD 72 million. Deferred sales price receivables and other adjustments amounted to
USD 41 million.
In 2021, USD 66 million included USD 52 million net cash inflows from divestments in previous years, and a USD 14 million net cash inflow from a business divestment in 2021,
comprised of intangible assets.
In 2020, USD 49 million represented the net cash inflows from divestments in previous years.
Notes 2 and 24 provide further information regarding acquisitions and divestments of businesses. All acquisitions
were for cash.
23.6) Reconciliation of liabilities arising from financing activities
Current
financial
debts and
Non-current
derivative
financial
financial
Non-current
Current lease
(USD millions) debts
instruments
lease liabilities
liabilities
January ,
Increase in non-current financial debts
Repayments of the current portion of non-current financial debts
–
Change in current financial debts
Payments of lease liabilities
–
Interest payments for amounts included in lease liabilities
classified as cash flows from operating activities
–
New, modified and terminated leases, net
Impact of acquisitions and divestments of businesses, net
Changes in fair values, lease interest and other changes, net
–
Amortization of bonds discount
Currency translation eects –
–
–
–
Reclassification from non-current to current, net –
–
December ,
1
Change in current financial debts included net cash outflows from interest-bearing accounts of employees payable on demand amounting to USD 1.7 billion. See Note 21.

Notes to the Novartis Groupconsolidatedfinancial statements
F-52
Current
financial
debts and
Non-current
derivative
financial
financial
Non-current
Current lease
(USD millions) debts
instruments
lease liabilities
liabilities
January ,
Increase in non-current financial debts
Repayments of the current portion of non-current financial debts
–
Change in current financial debts
–
Payments of lease liabilities, net
–
Interest payments for amounts included in lease liabilities
classified as cash flows from operating activities
–
New, modified and terminated leases, net
Impact of acquisitions of businesses
Changes in fair values, lease interest and other changes, net
–
Amortization of bonds discount
Currency translation eects –
–
–
–
Reclassification from non-current to current, net –
–
December ,
Current
financial
debts and
Non-current
derivative
financial
financial
Non-current
Current lease
(USD millions) debts
instruments
lease liabilities
liabilities
January ,
Increase in non-current financial debts
Repayments of the current portion of non-current financial debts
–
Change in current financial debts
Payments of lease liabilities, net
–
Interest payments for amounts included in lease liabilities
classified as cash flows from operating activities
–
New, modified and terminated leases, net
Impact of acquisitions of businesses
Changes in fair values, lease interest and other changes, net –
–
Amortization of bonds discount
Currency translation eects
Reclassification from non-current to current, net –
–
December ,
23.7) Supplemental disclosures related to the Alcon business distributed to Novartis AG
shareholders
In 2020, net cash flows used in investing activities from discontinued operations of USD 127 million included the
investing activities of the Alcon business, which was spun-o to Novartis AG shareholders on April 8, 2019, and
cash outflows for transaction-related expenditures attributable to the series of portfolio transformation transac-
tions completed in 2015.
In 2020, net cash flows used in financing activities from discontinued operations of USD 50 million were for
transaction cost payments directly attributable to the distribution (spin-o) of the Alcon business to Novartis AG
shareholders on April 8, 2019.

Notes to the Novartis Groupconsolidatedfinancial statements
F-53
24. Acquisitions of businesses
Fair value of assets and liabilities arising from acquisitions of businesses:
(USD millions)
Property, plant and equipment
Right-of-use assets
Currently marketed products
Acquired research and development
Other intangible assets
Deferred tax assets
Non-current financial and other assets
Inventories
Trade receivables and financial and other current assets
Cash and cash equivalents
Deferred tax liabilities –
–
–
Current and non-current financial debts
–
–
Current and non-current lease liabilities –
–
Trade payables and other liabilities –
–
–
Net identifiable assets acquired
Acquired cash and cash equivalents –
–
–
Non-controlling interests
–
Goodwill
Net assets recognized as a result of acquisitions of businesses
Note 2 details significant acquisitions of businesses, spe-
cifically of Gyroscope in 2022, the cephalosporin anti-
biotics business from GSK in 2021; and of the The
Medicines Company and the Japanese business of AGI
in 2020. The goodwill arising out of these acquisitions is
attributable to the buyer-specific synergies, the assem-
bled workforce, and the accounting for deferred tax lia-
bilities on the acquired assets. In 2022, no goodwill (2021:
USD 107 million; 2020: USD 74 million) is tax deductible.
25. Post-employment benefits for employees
Defined benefit plans
In addition to the legally required social security schemes,
the Group has numerous independent pension and other
post-employment benefit plans. In most cases, these
plans are externally funded in entities that are legally
separate from the Group. For certain Group companies,
however, no independent plan assets exist for the pen-
sion and other post-employment benefit obligations of
employees. In these cases, the related unfunded liability
is included in the balance sheet. The defined benefit obli-
gations (DBOs) of all major pension and other post-em-
ployment benefit plans are reappraised annually by inde-
pendent actuaries. Plan assets are recognized at fair
value. The major plans are based in Switzerland, the
United States, the United Kingdom, Germany and Japan,
which represent 95% of the Group’s total DBO for pen-
sion plans. Details of the plans in the two most signifi-
cant countries, Switzerland and the United States, which
represent 83% of the Group’s total DBO for post-em-
ployment benefit plans, are provided below.
Swiss-based pension plans represent the most sig-
nificant portion of the Group’s total DBO and plan assets.
For the active insured members the benefits are linked
to contributions paid into the plan, interest credits
granted and conversion rates applied.
All benefits granted under Swiss-based pension
plans are vested, and Swiss legislation prescribes that
the employer has to contribute a fixed percentage of an
employee’s pay to an external pension fund. Additional
employer contributions may be required whenever the
plan’s statutory funding ratio falls below a certain level.
The employee also contributes to the plan. The pension
plans are run by separate legal entities, each governed
by a board of trustees that – for the principal plans – con-
sists of representatives nominated by Novartis and the
active insured employees. The boards of trustees are
responsible for the plan design and asset investment
strategy.

Notes to the Novartis Groupconsolidatedfinancial statements
F-54
In December 2020, the Board of Trustees of the
Novartis Swiss Pension Fund agreed to adjust the annu-
ity conversion rate at retirement with eect from Janu-
ary 1, 2022. This amendment did not aect existing pen-
sioners, and its impact on existing plan participants will
be mitigated by way of defined compensatory measures.
This amendment resulted in a net pre-tax curtailment
gain of USD 101 million (CHF 90 million) recognized in
2020.
The United States pension plans represent the sec-
ond-largest component of the Group’s total DBO and
plan assets. The principal plans (Qualified Plans) are
funded, whereas plans providing additional benefits for
executives (Restoration Plans) are unfunded. Employer
contributions are required for Qualified Plans whenever
the statutory funding ratio falls below a certain level.
Furthermore, in certain countries, employees are cov-
ered under other post-employment benefit plans and
post-retirement medical plans.
In the US, other post-employment benefit plans con-
sist primarily of post-employment healthcare benefits,
which have been closed to new members since 2015.
Part of the costs of these plans is reimbursable under
the Medicare Prescription Drug, Improvement, and Mod-
ernization Act of 2003. There is no statutory funding
requirement for these plans. The Group is funding these
plans to the extent that it is tax ecient.
The following tables are a summary of the funded and unfunded defined benefit obligation for pension and other
post-employment benefit plans of employees at December 31, 2022 and 2021:
Pension plans Other post-employment
benefit plans
(USD millions)
Benefit obligation at January
Current service cost
Interest cost
Past service costs and settlements –
–
Administrative expenses
Remeasurement gains arising from changes in financial assumptions
–
–
–
–
Remeasurement (gains)/losses arising from changes in demographic assumptions –
–
Experience-related remeasurement losses/(gains)
–
–
Currency translation eects –
–
–
–
Benefit payments –
–
–
–
Contributions of employees
Eect of acquisitions, divestments or transfers –
Benefit obligation at December
Fair value of plan assets at January
Interest income
Return on plan assets excluding interest income –
–
Currency translation eects –
–
Novartis Group contributions
Contributions of employees
Settlements –
–
Benefit payments –
–
–
–
Eect of acquisitions, divestments or transfers
Fair value of plan assets at December
Funded status
–
–
–
Limitation on recognition of fund surplus at January –
–
Change in limitation on recognition of fund surplus –
–
Currency translation eects –
Interest income on limitation of fund surplus –
–
Limitation on recognition of fund surplus at December
–
–
Net liability in the balance sheet at December –
–
–
–
1
The remeasurement gains arising from changes in financial assumptions is driven mainly by changes in the actuarial discount rates used to determine the benefit obligation.
2
As of December 31, 2022, the most significant pension plans where the asset ceiling was required to be applied were in Switzerland and amounted to USD 2 587 million.

Notes to the Novartis Groupconsolidatedfinancial statements
F-55
The reconciliation of the net liability from January 1 to December 31 is as follows:
Pension plans Other post-employment
benefit plans
(USD millions)
Net liability at January –
–
–
–
Current service cost –
–
–
–
Net interest expense –
–
–
–
Administrative expenses –
–
Past service costs and settlements
–
–
Remeasurements
Currency translation eects
Novartis Group contributions
Eect of acquisitions, divestments or transfers
–
Change in limitation on recognition of fund surplus –
–
Net liability at December –
–
–
–
Amounts recognized in the consolidated balance sheet
Prepaid benefit cost
Accrued benefit liability –
–
–
–
The following table shows a breakdown of the DBO for pension plans by geography and type of member, and the
breakdown of plan assets into the geographical locations in which they are held:
2022 2021
United
Rest of
United
Rest of
(USD millions) Switzerland
States
the world
Total
Switzerland
States
the world
Total
Benefit obligation at December
Thereof unfunded
556
363
919
688
439
1 127
By type of member
Active
Deferred pensioners
Pensioners
Fair value of plan assets at December
Funded status
–
–
–
–
–
The following table shows a breakdown of the DBO for other post-employment benefit plans by geography and
type of member, and the breakdown of plan assets into the geographical locations in which they are held:
2022 2021
United
Rest of
United
Rest of
(USD millions) States
the world
Total
States
the world
Total
Benefit obligation at December
Thereof unfunded 286
76
362
400
87
487
By type of member
Active
Deferred pensioners
Pensioners
Fair value of plan assets at December
Funded status –
–
–
–
–
–
Notes to the Novartis Groupconsolidatedfinancial statements
F-56
The following table shows the principal weighted average actuarial assumptions used for calculating defined ben-
efit plans and other post- employment benefits of employees:
Pension plans Other post-employment
benefit plans
Weighted average assumptions used to determine
benefit obligations at December
Discount rate
Expected rate of pension increase
Expected rate of salary increase
Interest on savings account
Current average life expectancy
for a -year-old male in years
Current average life expectancy
for a -year-old female in years
Changes in the aforementioned actuarial assumptions
can result in significant volatility in the accounting for the
Group’s pension plans in the consolidated financial state-
ments. This can result in substantial changes in the
Group’s other comprehensive income, long-term liabili-
ties and prepaid pension assets.
The DBO is significantly impacted by assumptions
regarding the rate that is used to discount the actuari-
ally determined post-employment benefit liability. This
rate is based on yields of high-quality corporate bonds
in the country of the plan. Decreasing corporate bond
yields decrease the discount rate, so that the DBO
increases and the funded status decreases.
In Switzerland, an increase in the DBO due to lower
discount rates is slightly oset by lower future benefits
expected to be paid on the employee’s savings account
where the assumption on interest accrued often changes
broadly in line with the discount rate.
The impact of decreasing interest rates on a plan’s
assets is more dicult to predict. A significant part of
the plan assets is invested in bonds. Bond values usually
rise when interest rates decrease and may therefore par-
tially compensate for the decrease in the funded status.
Furthermore, pension assets also include significant
holdings of equity instruments. Share prices usually tend
to rise when interest rates decrease and therefore often
counteract the negative impact of the rising defined ben-
efit obligation on the funded status (although the
correlation of interest rates with equities is not as strong
as with bonds, especially in the short term).
The expected rate for pension increases significantly
aects the DBO of most plans in Switzerland, Germany
and the United Kingdom. Such pension increases also
decrease the funded status, although there is no strong
correlation between the value of the plan assets and
pension/inflation increases.
Assumptions regarding life expectancy significantly
impact the DBO. An increase in longevity increases the
DBO. There is no osetting impact from the plan assets,
as no longevity bonds or swaps are held by the pension
funds. The Group’s actuaries use mortality tables which
take into account historic patterns and expected
changes, such as further increases in longevity.
In 2022 the mortality assumptions used for the pen-
sion plans in Switzerland were based on BVG 2020
tables with future improvements based on the BVG gen-
erational model. In US for the Pension and Postretire-
ment Medical Benefit Plans, the Society of Actuaries Pri-
2012 mortality tables with generational improvements
based on Scale MP-2021 are used.
The following table shows the sensitivity of the
defined benefit pension obligation to the principal actu-
arial assumptions for the major plans in Switzerland, the
United States, the United Kingdom, Germany and Japan
on an aggregated basis:
Change in
Change in
year-end defined
year-end defined
benefit pension
benefit pension
(USD millions) obligation
obligation
basis point increase in discount rate –
–
basis point decrease in discount rate
One-year increase in life expectancy
basis point increase in rate of pension increase
basis point decrease in rate of pension increase –
–
basis point increase of interest on savings account
basis point decrease of interest on savings account –
–
basis point increase in rate of salary increase
basis point decrease in rate of salary increase –
–
Notes to the Novartis Groupconsolidatedfinancial statements
F-57
The healthcare cost trend rate assumptions used for
other post- employment benefits are as follows:
Healthcare cost trend rate
assumed for next year
Rate to which the cost trend
rate is assumed to decline
Year that the rate reaches
the ultimate trend rate
The following table shows the weighted average plan
asset allocation of funded defined benefit pension plans
at December 31, 2022 and2021:
Pension plans
Long-term
Long-term
target
target
(as a percentage) minimum
maximum
Equity securities
Debt securities
Real estate
Alternative investments
Cash and other investments
Total
Cash and most of the equity and debt securities have a
quoted market price in an active market. Real estate and
alternative investments, which include hedge fund, pri-
vate equity, infrastructure and commodity investments,
usually have a quoted market price or a regularly updated
net asset value.
The strategic allocation of assets of the dierent pen-
sion plans is determined with the objective of achieving
an investment return that, together with the contributions
paid by the Group and its employees, is sucient to main-
tain reasonable control over the various funding risks of
the plans. Based upon the market and economic envi-
ronments, actual asset allocations may temporarily be
permitted to deviate from policy targets. The asset allo-
cation currently includes investments in shares of
Novartis AG as per the below table:
December ,
December ,
Investment in shares of Novartis AG
Number of shares (in millions)
Market value (in USD billions)
The weighted average duration of the defined benefit
pension obligation is 11.8 years (2021: 14.9 years).
The Group’s ordinary contribution to the various pen-
sion plans is based on the rules of each plan. Additional
contributions are made whenever this is required by stat-
ute or law (i.e., usually when statutory funding levels fall
below predetermined thresholds). The only significant
plans that require additional funding are those in the
United Kingdom and Germany.
The expected future cash flows in respect of pension
and other post-employment benefit plans at December
31, 2022, were as follows:
Other post-
employment
(USD millions) Pension plans
benefit plans
Novartis Group contributions
(estimated)
Expected future benefit payments
–
Defined contribution plans
In many subsidiaries, employees are covered by defined
contribution plans. Contributions charged to the consol-
idated income statement for the defined contribution
plans were:
(USD millions)
Contributions for defined contribution plans
The Group’s total personnel costs amounted to USD 14.9
billion in 2022.
Notes to the Novartis Groupconsolidatedfinancial statements
F-58
26. Equity-based participation plans for employees
The expense related to all equity-based participation
plans and the liabilities arising from equity-based pay-
ment transactions were as follows:
(USD millions)
Expense related to equity-based
participation plans
Liabilities arising from equity-based
payment transactions
Equity-based participation plans can be separated into
the following plans:
Annual Incentive
The Annual Incentive for the Novartis Group CEO and
other Executive Committee members (ECN) is paid 50%
in cash and 50% in Novartis restricted shares (RSs) or
restricted share units (RSUs). For the Novartis Top Lead-
ers (NTLs), the Annual Incentive is paid 70% in cash and
30% in RSs or RSUs. Both the ECN and NTLs can opt
to invest up to the maximum cash portion of their Annual
Incentive to receive further RSs or RSUs. Any cash is
paid out during March in the year following the end of
the performance period, and the shares are granted
during January in the year following the end of the per-
formance period.
Employee share savings plan
Novartis operates employee share savings and purchase
plans in certain countries. The most significant is
described below.
The Employee Share Ownership Plan (ESOP) in Swit-
zerland oers participants to choose to receive their
Annual Incentive (i) 100% in shares, (ii) 50% in shares
and 50% in cash, or (iii) 100% in cash. After expiration
of a three-year holding period for Novartis shares
invested under the ESOP, participants will receive one
matching share for every two invested shares. Employ-
ees eligible for the equity plan “Select” are not eligible
to receive ESOP matching shares. The Novartis Group
CEO, the other Executive Committee members and the
NTLs are not eligible to participate in this plan.
Novartis Employee share purchase
plan
In 2022 Novartis started to grant shares under the
Employee Share Purchase Plan. The plan enables
employees to voluntarily purchase Novartis shares
through payroll deductions at a discounted price. While
the plan is global in scope, the first phase covers: North
America (the US, Puerto Rico and Canada). The shares
are not subject to a vesting period.
Novartis equity plan “Select”
The equity plan “Select” is a global equity incentive plan
under which eligible employees may annually be awarded
a grant subject to a three-year, and for selected units a
four-year, staggered vesting period. No awards are
granted for performance ratings below a certain thresh-
old. Executive Committee members and NTLs are not
eligible to participate in the equity plan “Select.”
The equity plan “Select” currently allows participants
employed and living in Switzerland to choose the form
of their equity compensation in RSs or RSUs. In all other
jurisdictions, RSs or RSUs are granted unilaterally. Until
2013, participants could also choose to receive part or
the entire grant in the form of tradable share options.
Tradable share options expire on their 10
th
anniver-
sary from the grant date, meaning all outstanding options
exercisable at December 31, 2022, will expire in January
2023. Each tradable share option entitles the holder to
purchase after vesting (and before the 10
th
anniversary
from the grant date) one Novartis share at a stated exer-
cise price that equals the closing market price of the
underlying share at the grant date. As the exercise price
does not reflect the decrease in the Novartis share due
to the Alcon spin, one-fifth of an Alcon share will also be
awarded to the option holder upon exercise.
Options under Novartis equity plan “Select”
outside North America
The following table shows the activity associated with
the share options during the period. The weighted aver-
age prices in the table below are translated from Swiss
francs into USD at historical rates.
2022 2021
Weighted
Weighted
average
average
exercise
exercise
Options
price
Options
price
(millions)
(USD)
(millions)
(USD)
Options outstanding
at January
Sold or exercised –
–
Outstanding at December
Exercisable at December
All share options were granted at an exercise price that
was equal to the closing market price of the Group’s
shares at the grant date. The weighted average share
price at the dates of sale or exercise was USD86.1.
Options under Novartis equity plan “Select” for
North America
The following table shows the activity associated with
the ADR options during the period:

Notes to the Novartis Groupconsolidatedfinancial statements
F-59
2022 2021
Weighted
Weighted
average
average
ADR
exercise
ADR
exercise
options
price
options
price
(millions)
(USD)
(millions)
(USD)
Options outstanding
at January
Sold or exercised –
–
Outstanding at December
Exercisable at December
All ADR options were granted at an exercise price that
was equal to the closing market price of the ADRs at the
grant date. The weighted average ADR price at the dates
of sale or exercise was USD89.1.
Long-Term Performance Plan
The Long-Term Performance Plan (LTPP) is an equity plan
for the ECN, the NTLs and employees of Group units with
specific targets.
Participants are granted a target number of perfor-
mance share units (PSUs) at the beginning of every per-
formance period, which are converted into unrestricted
Novartis shares after the performance period. The actual
payout depends on the achievement of the performance
measures and ranges between 0% and 200% of the
granted amount. PSUs granted under the LTPP do not
carry voting rights, but do carry dividend equivalents that
are paid in unrestricted Novartis shares at the end of the
performance period.
The LTPP awards are subject to a three-year perfor-
mance and vesting period. Until 2018, the performance
criteria were based on Novartis internal performance
metrics. For LTPP awards starting in 2019, following the
combination of the two LTPP and Long-Term Relative
Performance Plan (LTRPP), the performance criteria are
based on both Novartis internal performance metrics and
variables that can be observed in the market, which is
the ranking of the Novartis total shareholder return (TSR)
relative to a global healthcare peer group of 14 other
companies, over rolling three-year performance periods.
TSR for Novartis and the peer companies is calcu-
lated as the change in the company share price, which
is translated to USD at the relevant exchange rate, includ-
ing the reinvestment return of dividends, over the three-
year performance period. The calculation is based on
Bloomberg standard published TSR data, which is pub-
licly available. The position of Novartis in the peer group
determines the payout range based on a payout matrix.
Long-Term Relative Performance
Plan
The LTRPP was an equity plan for the Novartis ECN and
NTLs and the awards were subject to a three-year per-
formance and vesting period. The last grant under this
plan was made in 2018. The LTRPP performance crite-
ria were based on variables that could be observed in
the market, which was the ranking of the Novartis TSR
relative to a global healthcare peer group of 14 other
companies, over rolling three-year performance periods.
The TSR for Novartis and the peer companies was cal-
culated as described in the LTPP section above.
Other share awards
Selected employees may exceptionally receive Special
Share Awards of RSs or RSUs. These Special Share
Awards provide an opportunity to reward outstanding
achievements or exceptional performance, and aim to
retain key contributors. They are based on a formal inter-
nal selection process, through which the individual per-
formance of each candidate is thoroughly assessed at
several management levels. Special Share Awards had
a minimum three-year vesting period before 2021 and
mainly three years thereafter. In exceptional circum-
stances, Special Share Awards may be awarded to
attract special expertise and new talents to the
organization. Externally recruited ECN members are eli-
gible only for special awards that are “buyouts” in the
case that it is to replace equity forfeited with their for-
mer employer. The equity is provided on a like-for-like
basis as the forfeited equity, at the same value with the
same vesting period, and with or without a performance
condition.
Worldwide, employees at dierent levels in the orga-
nization were awarded RSs and RSUs in 2022, 2021 and
2020.
In addition, in 2022, 2021 and 2020, Board members
received unrestricted shares as part of their regular com-
pensation.

Notes to the Novartis Groupconsolidatedfinancial statements
F-60
Summary of share grants
The table below provides a summary of share grants (shares, RSs, RSUs and PSUs) for all plans:
2022 2021
Weighted
Weighted
Number
average fair
Number
average fair
of shares
value at grant
of shares
value at grant
in millions
date in USD
in millions
date in USD
Annual Incentive
– RSU
– Restricted shares
Share savings plans
– RSU
– Shares
Novartis Employee Share Purchase Plan
Select North America (RSU)
Select outside North America
– RSU
– Restricted shares
Long-Term Performance Plan (PSU)
Other share awards
– RSU
– Restricted shares
– Shares

Notes to the Novartis Groupconsolidatedfinancial statements
F-61
27. Transactions with related parties
Roche Holding AG
Novartis has two agreements with Genentech, Inc.,
United States (Genentech), and one agreement with
Spark Therapeutics, Inc., United States (Spark). Both
companies are subsidiaries of Roche Holding AG
(Roche), which were indirectly included in the consoli-
dated financial statements using equity accounting until
November 3, 2021, when Novartis entered into an agree-
ment with Roche to divest its 33.3% of Roche voting
shares. On December 6, 2021, Novartis divested its
investment in Roche, on which date Roche ceased to be
a related party (see Notes 2 and 4).
Lucentis
Novartis has licensed from Genentech/Roche the exclu-
sive rights to develop and market Lucentis outside the
United States for indications related to diseases of the
eye. Novartis pays royalties on the net sales to third par-
ties of Lucentis products outside the United States. From
January 1, 2021 until December 6, 2021, Lucentis sales
of USD2.0 billion (2020: USD1.9 billion) were recognized
by Novartis.
Xolair
Novartis and Genentech/Roche are co-promoting Xolair
in the United States, where Genentech/Roche records
all sales. Novartis records sales outside the United
States.
Novartis markets Xolair and records all sales and
related costs outside the United States as well as co-pro-
motion costs in the US. Genentech/Roche and Novartis
share the resulting profits from sales in the United States,
Europe and other countries, according to agreed prof-
it-sharing percentages. From January 1, 2021 until
December 6, 2021, Novartis recognized total sales of
Xolair of USD1.3 billion (2020: USD1.3 billion), including
sales to Genentech/Roche for the United States market.
Luxturna
In 2018, Novartis entered into an exclusive licensing and
commercialization agreement and a supply agreement
with Spark for Luxturna outside the United States. The
agreements include regulatory and sales milestones as
well as royalties payable to Spark on ex-US sales. On
December 17, 2019, Roche acquired Spark.
The net income for royalties, cost sharing and profit shar-
ing arising out of the Lucentis, Xolair and Luxturna agree-
ments with Roche totaled USD188 million from January
1, 2021 until December 6, 2021 (net income in 2020:
USD217 million).
Furthermore, Novartis has several patent license,
supply and distribution agreements with Roche.
Novartis Pension Fund
In 2018, a Group subsidiary provided an uncommitted
overnight credit facility to the Novartis Pension Fund,
Switzerland, for up to USD 500 million with interest at
the US Federal Funds Rate. This credit facility was not
utilized during the current and past years.
Executive Ocers and Non-Executive Directors compensation
At December 31, 2022, there were 11 Executive Com-
mittee members (“Executive Ocers”). During 2022, 5
Executive Ocers stepped down. At December 31,
2021, there were 12 Executive Ocers. During 2021, 3
Executive Ocers stepped down. At December 31,
2020, there were 13 Executive Ocers.
The total compensation for Executive Committee members and the 15 Non-Executive Directors (14 in 2021 and
14 in 2020) using the Group’s accounting policies for equity-based compensation and pension benefits was as fol-
lows:
Executive Ocers Non-Executive Directors Total
(USD millions)
Cash and other compensation
Post-employment benefits
Equity-based compensation
Total
During 2022, there was an increase in the IFRS compen-
sation expense for executive ocers compared to 2021,
driven by accelerated expenses (cash and other com-
pensation and equity-based compensation) required
under IFRS for the executive members who stepped
down in 2022, in accordance with their employment con-
tracts and the relevant incentive plan terms, compared
to the accelerated expenses due to executive ocers
who stepped down in 2021.

Notes to the Novartis Groupconsolidatedfinancial statements
F-62
During 2021, the IFRS compensation expense
decreased due to one role less at the ECN, and lower
cash and equity compensation attributable to former
ECN members, partially oset by the net increase of the
IFRS compensation expense of current ECN members.
The Annual Incentive award, which is fully included
in equity- based compensation even when paid out in
cash, is granted in January in the year following the
reporting period.
The disclosures on Board and executive compensa-
tion required by the Swiss Code of Obligations and in
accordance with the Swiss Ordinance against Excessive
Compensation in Stock Exchange Listed Companies are
shown in the Compensation Report of the Group.
Transactions with former members of the Board of
Directors
During 2022, 2021 and 2020, the following payments (or
waivers of claims) were made to former Board members
or to “persons closely” linked to them:
Currency
Dr. Krauer CHF
Dr. Alex Krauer, was an Honorary Chairman of Novartis
and was entitled to an amount of CHF 60 000 for annual
periods from one AGM to the next. This amount was fixed
in 1998 upon his departure from the Board in 1999. The
last payment under this arrangement was in 2021.
28. Commitments and contingent liabilities
Research and development
commitments
The Group has entered into long-term research and
development agreements with various institutions related
to intangible assets. These agreements provide for
potential milestone payments by Novartis, which are
dependent on successful clinical development, or meet-
ing specified sales targets, or other conditions which are
specified in the agreements.
As of December 31, 2022, the amount and estimated
timing of the Group’s commitments to make payments
under those agreements, which are shown without risk
adjustment and on an undiscounted basis, were as fol-
lows:
(USD millions)
Thereafter
Total
Commitments for capital calls
The Group holds investments in funds in which it has
committed to invest further upon future capital calls. As
of December 31, 2022, the total uncalled capital com-
mitments for the Group’s investments in funds amounts
to USD 83 million. Note 29 contains further information
on the Group’s investments in funds.
Other commitments
The Group has entered into various purchase commit-
ments for services and materials as well as for equip-
ment in the ordinary course of business. These commit-
ments are generally entered into at current market prices
and reflect normal business operations. For disclosure
of property, plant and equipment purchase commit-
ments, see Note 9.
Guarantees issued
The Group has issued guarantees to third parties in the
ordinary course of business, mostly for tax, customs or
other governmental agencies.
Contingent liabilities
Group companies have to observe the laws, government
orders and regulations of the country in which they
operate.
A number of Novartis companies are, and will likely
continue to be, subject to various legal proceedings and
investigations that arise from time to time, including pro-
ceedings regarding product liability; sales and market-
ing practices; commercial disputes; employment and
wrongful discharge; and antitrust, securities, health and
safety, environmental, tax, international trade, privacy
and intellectual property matters. As a result, the Group
may become subject to substantial liabilities that may
not be covered by insurance and that could aect our
business, financial position and reputation. While Novartis
does not believe that any of these legal proceedings will
have a material adverse eect on its financial position,
litigation is inherently unpredictable and large judgments
sometimes occur. As a consequence, Novartis may in
the future incur judgments or enter into settlements of

Notes to the Novartis Groupconsolidatedfinancial statements
F-63
claims that could have a material adverse eect on its
results of operations or cash flow.
Governments and regulatory authorities around the
world have been stepping up their compliance and law
enforcement activities in recent years in key areas,
including marketing practices, pricing, corruption, trade
restrictions, embargo legislation, insider trading, anti-
trust, cyber security and data privacy. Further, when one
government or regulatory authority undertakes an inves-
tigation, it is not uncommon for other governments or
regulators to undertake investigations regarding the
same or similar matters. Responding to such investiga-
tions is costly and requires an increasing amount of man-
agement’s time and attention. In addition, such investi-
gations may aect our reputation, create a risk of
potential exclusion from government reimbursement
programs in the United States and other countries, and
lead to (or arise from) litigation. These factors have con-
tributed to decisions by Novartis and other co mpanies
in the healthcare industry, when deemed in their interest,
to enter into settlement agreements with governmental
authorities around the world prior to any formal decision
by the authorities or a court. These government settle-
ments have involved and may in the future involve large
cash payments, sometimes in the hundreds of millions
of dollars or more, including the potential repayment of
amounts allegedly obtained improperly and other pen-
alties, including treble damages. In addition, settlements
of government healthcare fraud cases and antitrust
cases often require companies to enter into corporate
integrity agreements, which are intended to regulate
company behavior for a period of years. Our aliates
Novartis Corporation and Sandoz Inc. are parties to such
agreements, which will expire in 2025 and 2026, respec-
tively. Also, matters underlying governmental
investigations and settlements may be the subject of
separate private litigation.
While provisions have been made for probable out-
flows of economic resources, which management deems
to be reasonable or appropriate, there are uncertainties
connected with these estimates.
Note 20 contains additional information on these
matters.
A number of Group companies are involved in legal
proceedings concerning intellectual property rights. The
inherent unpredictability of such proceedings means
that there can be no assurances as to their ultimate out-
come. A negative result in any such proceeding could
potentially adversely aect the ability of certain Novartis
companies to sell their products, or require the payment
of substantial damages or royalties. The timing and the
outcome of legal proceedings and their potential finan-
cial eect are not predictable.
In the opinion of management, however, the outcome
of these actions will not materially aect the Group’s
financial position but could be material to the results of
operations or cash flow in a given period.
The Group’s potential environmental remediation lia-
bility is assessed based on a risk assessment and inves-
tigation of the various sites identified by the Group as at
risk for environmental remediation exposure. The Group’s
future remediation expenses are aected by a number
of uncertainties. These uncertainties include, but are not
limited to, the method and extent of remediation, the per-
centage of material attributable to the Group at the reme-
diation sites relative to that attributable to other parties,
and the financial capabilities of the other potentially
responsible parties.
Note 20 contains additional information on environ-
mental liabilities.

Notes to the Novartis Groupconsolidatedfinancial statements
F-64
29. Financial instruments–additional disclosures
The following tables show the carrying values of finan-
cial instruments by measurement category as of Decem-
ber 31, 2022 and 2021. Except for straight bonds (see
Note 19), the carrying values are equal to, or a reason-
able approximation of, the fair values.
2022
Financial
Financial
instruments at
instruments at
fair value
Other
Financial
fair value
through the
financial
instruments at
through other
consolidated
liabilities at
amortized
comprehensive
income
amortized
(USD millions) Note
costs
income
statement
costs
Cash and cash equivalents
Time deposits and short-term investments with original maturity more than days
Trade receivables
Other receivables and current assets
Marketable securities – debt securities
Long-term financial investments – equity securities
Long-term financial investments – debt securities
Long-term financial investments – fund investments
Long-term loans, advances, security deposits and other long-term receivables
Associated companies at fair value through profit and loss
Derivative financial instruments
Contingent consideration receivables /
Total financial assets
Bank and other short-term financial debt
Commercial paper
Straight bonds
Long-term liabilities to banks and other financial institutions
Trade payables
Contingent consideration liabilities (see Note /) and other financial liabilities
Derivative financial instruments
Lease liabilities
Total financial liabilities

Notes to the Novartis Groupconsolidatedfinancial statements
F-65
2021
Financial
Financial
instruments at
instruments at
fair value
Other
Financial
fair value
through the
financial
instruments at
through other
consolidated
liabilities at
amortized
comprehensive
income
amortized
(USD millions) Note
costs
income
statement
costs
Cash and cash equivalents
Time deposits and short-term investments with original maturity more than days
Trade receivables
Other receivables and current assets
Marketable securities – debt securities
Long-term financial investments – equity securities
Long-term financial investments – debt securities
Long-term financial investments – fund investments
Long-term loans, advances, security deposits and other long-term receivables
Associated companies at fair value through profit and loss
Derivative financial instruments
Contingent consideration receivables
Total financial assets
Interest-bearing accounts of employees payable on demand
Bank and other short-term financial debt
Commercial paper
Straight bonds
Long-term liabilities to banks and other financial institutions
Trade payables
Commitment for repurchase of own shares /
Contingent consideration liabilities (see Note /) and other financial liabilities
Derivative financial instruments
Lease liabilities
Total financial liabilities
1
Includes short-term highly rated government-backed debt securities, with an original maturity of three months or less
Derivative financial instruments
The following tables show the contract or underlying
principal amounts and fair values of derivative financial
instruments analyzed by type of contract at Decem-
ber31, 2022 and 2021. Contract or underlying principal
amounts indicate the gross volume of business outstand-
ing at the consolidated balance sheet date and do not
represent amounts at risk. The fair values are determined
by reference to market prices or standard pricing mod-
els that use observable market inputs at December
31,2022 and 2021.
Contract or underlying Positive fair values Negative fair values
principal amount
(USD millions)
Forward foreign exchange rate contracts
–
–
Commodity purchase contract
Options on equity securities
–
–
Total derivative financial instruments included in
marketable securities and in current financial debts
–
–
Notes to the Novartis Groupconsolidatedfinancial statements
F-66
The following table shows a breakdown by currency of the contract or underlying principal amount of derivative
financial instruments at December31,2022 and 2021:
2022
(USD millions) EUR
USD
Other
Total
Forward foreign exchange rate contracts
Commodity purchase contract
Options on equity securities
Total derivative financial instruments
2021
(USD millions) EUR
USD
Other
Total
Forward foreign exchange rate contracts
Commodity purchase contract
Options on equity securities
Total derivative financial instruments
Derivative financial instruments eective for hedge
accounting purposes
At the end of 2022 and 2021, there were no open hedg-
ing instruments for anticipated transactions.
Fair value by hierarchy
As required by IFRS, financial assets and liabilities
recorded at fair value in the consolidated financial state-
ments are categorized based upon the level of judgment
associated with the inputs used to measure their fair
value. There are three hierarchical levels, based on
increasing subjectivity associated with the inputs to
derive fair valuation for these assets and liabilities, which
are as follows:
The assets carried at Level 1 fair value are equity and
debt securities as well as fund investments listed in active
markets.
The assets generally included in Level 2 fair value
hierarchy are derivatives, and certain debt securities. The
liabilities generally included in this fair value hierarchy
consist of derivatives. These are valued using corrobo-
rated market data.
Level 3 inputs are unobservable for the asset or lia-
bility. The assets generally included in Level 3 fair value
hierarchy are various investments in funds and unquoted
equity security investments. Contingent consideration
and other financial liabilities carried at fair value are
included in this category.
2022
(USD millions) Level
Level
Level
Total
Financial assets
Marketable securities
Debt securities
Derivative financial instruments
Total marketable securities and derivative financial instruments at fair value
Current contingent consideration receivables
Long-term financial investments
Debt and equity securities
Fund investments
Non-current contingent consideration receivables
Total long-term financial investments at fair value
Associated companies at fair value through profit and loss
Financial liabilities
Current contingent consideration liabilities
–
–
Derivative financial instruments
–
–
Total current financial liabilities at fair values
–
–
–
Non-current contingent consideration liabilities
–
–
Other financial liabilities
–
–
Total non-current financial liabilities at fair value
–
–

Notes to the Novartis Groupconsolidatedfinancial statements
F-67
2021
(USD millions) Level
Level
Level
Total
Financial assets
Cash and cash equivalents
Debt securities
Total cash and cash equivalents at fair value
Marketable securities and derivative financial instruments
Debt securities
Derivative financial instruments
Total marketable securities and derivative financial instruments at fair value
Long-term financial investments
Debt and equity securities
Fund investments
Contingent consideration receivables
Total long-term financial investments at fair value
Associated companies at fair value through profit and loss
Financial liabilities
Contingent consideration payables
–
–
Derivative financial instruments
–
–
Other financial liabilities
–
–
Total financial liabilities at fair value
–
–
–
1
Includes short-term highly rated government-backed debt securities, with an original maturity of three months or less
The change in carrying values associated with Level 3 financial instruments, using significant unobservable inputs
during the year ended December 31, is set forth below:
2022
Associated
companies at
Long-term
Contingent
Contingent
Other
fair value through
Fund
financial
consideration
consideration
financial
(USD millions) profit and loss
investments
investments
receivables
liabilities
liabiltiies
January
–
–
Fair value gains and other adjustments, including from divestments
recognized in the consolidated income statement
Fair value losses (including impairments and amortizations) and
other adjustments recognized in the consolidated income statement –
–
–
–
–
Fair value adjustments recognized in the consolidated statement
of comprehensive income, including currency translation eects
Purchases
–
–
Cash receipts and payments
–
Disposals
–
–
Reclassification –
–
–
December
–
–
Total of fair value gains and losses recognized
in the consolidated income statement for assets
and liabilities held at December , –
–
–
–

Notes to the Novartis Groupconsolidatedfinancial statements
F-68
2021
Associated
companies at
Long-term
Contingent
Contingent
fair value through
Fund
financial
consideration
consideration
(USD millions) profit and loss
investments
investments
receivables
payables
January
–
Fair value gains and other adjustments, including from divestments
recognized in the consolidated income statement
Fair value losses (including impairments and amortizations) and
other adjustments recognized in the consolidated income statement –
–
–
–
–
Fair value adjustments recognized in the consolidated statement
of comprehensive income, including currency translation eects –
–
–
Purchases
–
Cash receipts and payments
–
Disposals –
–
–
Reclassification
–
–
December
–
Total of fair value gains and losses recognized
in the consolidated income statement for assets
and liabilities held at December , –
–
During 2022, there was one transfer of equity securities
from Level 3 to Level 1 for USD44 million (2021: USD73
million), due to Initial Public Oering of the invested com-
pany. During 2022, there were no transfers of equity
securities from Level 1 to Level 3 due to de-listing (2021:
USD 29 million).
Realized gains and losses associated with Level 3
long-term financial investments measured at fair value
through the consolidated income statement are recorded
in the consolidated income statement under “Other
income” or “Other expense,” respectively. Realized gains
and losses associated with Level 3 long-term financial
investments measured at fair value through other com-
prehensive income are not recycled through the consol-
idated income statement but are instead reclassified to
retained earnings.
During the year, the net loss and net gain recorded
on associated companies, fund investments and long-
term financial investments at fair value through profit and
loss were USD 316 million and USD 55 million, respec-
tively.
To determine the fair value of a contingent
consideration, various unobservable inputs are used. A
change in these inputs might result in a significantly
higher or lower fair value measurement. The inputs used
are, among others, the probability of success, sales fore-
cast and assumptions regarding the discount rate and
timing and dierent scenarios of triggering events. The
inputs are interrelated. The significance and usage of
these inputs to each contingent consideration may vary
due to dierences in the timing and triggering events for
payments or in the nature of the asset related to the con-
tingent consideration.
If the most significant parameters for the Level3 input
were to change by 10% positively or negatively, or where
the probability of success (POS) is the most significant
input parameter, 10% were added or deducted from the
applied probability of success, for contingent consider-
ation payables and contingent consideration receivables,
this would change the amounts recorded in the 2022
consolidated income statement by USD154 million and
USD140 million, respectively.
Equity securities measured at fair
value through other comprehensive
income
Equity securities held as strategic investments, typically
held outside the Novartis Venture Fund, are generally
designated at date of acquisition as financial assets val-
ued at fair value through other comprehensive income
with no subsequent recycling through profit and loss.
These are made up of individually non-significant invest-
ments. At December 31, 2022, the Group holds 65 non-
listed equity securities (December 31, 2021: 60) and 46
listed equity securities (December 31, 2021: 40) in this
category with the following fair values:
(USD millions)
Listed equity securities
Non-listed equity securities
Total equity securities
During 2022 and 2021, dividends received from these
equity securities were insignificant. In 2022, in accor-
dance with the consolidated foundations Alcon Inc.
shares divestment plans, Alcon Inc. shares with a fair
value of USD22 million were sold (2021: USD 9 million),
and the USD7 million gain on disposal (2021: USD 1 mil-
lion gain) was transferred from other comprehensive
income to retained earnings during 2022. In addition, in
2022, equity securities that were no longer considered
strategic, with a fair value of USD3 million (2021: USD254
million), were sold, and the USD3 million loss on disposal
(2021: USD211 million gain) was transferred from other
comprehensive income to retained earnings (see Note
8).

Notes to the Novartis Groupconsolidatedfinancial statements
F-69
Nature and extent of risks arising
from financial instruments
Market risk
Market risk in general comprises currency risk, interest
rate risk and price risk, such as commodity and equity
prices. Novartis is exposed to market risk, primarily
related to foreign currency exchange rates, interest rates
and the market value of the investments. The Group
actively monitors and seeks to reduce, where it deems
it appropriate to do so, fluctuations in these exposures.
It is the Group’s policy and practice to enter into a vari-
ety of derivative financial instruments to manage the vol-
atility of these exposures. It does not enter into any finan-
cial transactions containing a risk that cannot be
quantified at the time the transaction is concluded. In
addition, it does not sell short assets it does not have, or
does not know it will have, in the future. The Group only
sells existing assets or enters into transactions and
future transactions (in the case of anticipatory hedges)
that it confidently expects it will have in the future, based
on past experience.
Foreign currency exchange rate risk
The Group uses the US dollar as its reporting currency.
As a result, the Group is exposed to foreign currency
exchange movements, primarily in European, Japanese
and emerging market currencies. Fluctuations in the
exchange rates between the US dollar and other curren-
cies can have a significant eect on both the Group’s
results of operations, including reported sales and earn-
ings, as well as on the reported value of our assets, lia-
bilities and cash flows. This, in turn, may significantly
aect the comparability of period-to-period results of
operations.
Because our expenditures in Swiss francs are sig-
nificantly higher than our revenues in Swiss francs, vol-
atility in the value of the Swiss franc can have a signifi-
cant impact on the reported value of our earnings, assets
and liabilities, and the timing and extent of such volatility
can be dicult to predict.
There is also a risk that certain countries could expe-
rience a devaluation of their currency. If this occurs, it
could impact the eective prices we would be able to
charge for our products and also have an adverse impact
on both our consolidated income statement and balance
sheet.
Subsidiaries whose functional currencies have expe-
rienced a cumulative inflation rate of more than 100%
over the past three years apply the principles of IAS 29
“Financial reporting in Hyperinflationary Economies.”
The hyperinflationary economies in which Novartis oper
-
ates are Argentina, Venezuela and Turkey. Venezuela and
Argentina were hyperinflationary for all periods pre-
sented, and Turkey became hyperinflationary eective
May 1, 2022, requiring retroactive implementation of
hyperinflation accounting as of January 1, 2022. The
impacts of applying IAS 29 were not significant in all
years presented.
The Group manages its global currency exposure by
engaging in hedging transactions where management
deems appropriate. Novartis may enter into various con-
tracts that reflect the changes in the value of foreign cur-
rency exchange rates to preserve the value of assets,
commitments and anticipated transactions. Novartis also
uses forward contracts and may enter into foreign cur-
rency option contracts to hedge.
Net investments in subsidiaries in foreign countries
are long-term investments. Their fair value changes
through movements of foreign currency exchange rates.
The Group has designated a certain portion of its long-
term euro-denominated straight bonds, maturing in
2028, as hedges of the translation risk arising on certain
of these net investments in foreign operations with euro
functional currency. As of December 31, 2022, long-term
financial debt with a carrying amount of EUR1.8 billion
(USD2.0 billion; December 31, 2021: USD 2.1 billion), has
been designated as a hedge instrument. During 2022,
USD91 million of net of taxes unrealized income (2021:
USD 216 million) was recognized in other comprehen-
sive income and accumulated in currency translation
eects in relation with this net investment hedge. The
hedge remained eective since inception, and no amount
was recognized in the consolidated income statement
in 2022, 2021 and 2020.
Commodity price risk
The Group has only a very limited exposure to price risk
related to anticipated purchases of certain commodities
used as raw materials by the Group’s businesses. A
change in those prices may alter the gross margin of a
specific business, but generally by not more than 10% of
the margin and thus below the Group’s risk management
tolerance levels. Accordingly, the Group does not enter
into significant commodity futures, forward or option
contracts to manage fluctuations in prices of anticipated
purchases.
Interest rate risk
The Group addresses its net exposure to interest rate
risk mainly through the ratio of its fixed-rate financial
debt to variable-rate financial debt contained in its total
financial debt portfolio. To manage this mix, Novartis may
enter into interest rate swap agreements, in which it
exchanges periodic payments based on a notional
amount and agreed-upon fixed and variable interest
rates.
Equity risk
The Group may purchase equities as investments of its
liquid funds. As a policy, it limits its holdings in an unre-
lated company to less than 5% of its liquid funds. Poten-
tial investments are thoroughly analyzed. Call options
are written on equities that the Group owns, and put
options are written on equities that the Group wants to
buy and for which cash is available.
Credit risk
Credit risks arise from the possibility that customers may
not be able to settle their obligations as agreed. To man-
age this risk, the Group periodically assesses country
and customer credit risk, assigns individual credit limits,
and takes actions to mitigate credit risk where appropri-
ate (for example payment guarantees, credit insurance
and factoring).
The provisions for expected credit losses for cus-
tomers are based on a forward-looking expected credit
loss, which includes possible default events on the trade

Notes to the Novartis Groupconsolidatedfinancial statements
F-70
receivables over the entire holding period of the trade
receivables.
In measuring the expected credit losses, trade receiv-
ables are grouped based on shared credit risk charac-
teristics (such as private versus public receivables) and
days past due. In determining the expected credit loss
rates, the Group considers current and forward-looking
macroeconomic factors that may aect the ability of the
customers to settle the receivables, and historical loss
rates for each category of customers.
The Group’s largest customer accounted for approx-
imately 16% of net sales to third parties, and the second
largest and third largest customers accounted for 11%
and 7% of net sales to third parties, respectively (2021:
17%, 11% and 6%, respectively; 2020: 17%, 11% and 6%,
respectively).
The highest amounts of trade receivables outstand-
ing were for these same three customers and amounted
to 16%, 14% and 7%, respectively, of the Group’s trade
receivables at December 31, 2022 (2021: 16%, 12% and
7%, respectively). There is no other significant concen-
tration of customer credit risk.
Counterparty risk
Counterparty risk encompasses issuer risk on market-
able securities and money market instruments; credit risk
on cash, time deposits and derivatives; as well as settle-
ment risk for dierent instruments. Issuer risk is reduced
by only buying securities that are at least A- rated. Coun-
terparty credit risk and settlement risk are reduced by a
policy of entering into transactions with counterparties
(banks or financial institutions) that feature a strong
credit rating. Exposure to these risks is closely moni-
tored and kept within predetermined parameters. The
limits are regularly assessed and determined based upon
credit analysis, including financial statement and capital
adequacy ratio reviews. In addition, reverse repurchas-
ing agreements are contracted, and Novartis has entered
into credit support agreements with various banks for
derivative transactions. To further reduce the settlement
risk, the Group has implemented a multi-currency pay-
ment system, Continuous Linked Settlement (CLS), pro-
viding multilateral netting (payment-versus-payment set-
tlement) of cash flows from foreign exchange
transactions.
The Group’s cash and cash equivalents are held with
major regulated financial institutions; the three largest
ones hold approximately 13.2%, 9.2% and 6.8%, respec-
tively (2021: 9.7%, 9.7% and 7.6%, respectively). As of
December 31, 2021, the Group’s cash and cash equiva-
lents also included short-term highly rated govern-
ment-backed debt securities, with an original maturity of
three months or less, for approximately 16% (2022: nil).
The Group does not expect any losses from non-per
-
formance by these counterparties and does not have any
significant grouping of exposures to financial sector or
country risk.
Liquidity risk
Liquidity risk is defined as the risk that the Group could
not be able to settle or meet its obligations associated
with financial liabilities that are settled by delivering cash
or another financial asset. Group Treasury is responsi-
ble for liquidity, funding and settlement management. In
addition, liquidity and funding risks, and related pro-
cesses and policies, are overseen by management.
Novartis manages its liquidity risk on a consolidated
basis according to business needs and tax, capital or
regulatory considerations, if applicable, through numer-
ous sources of financing in order to maintain flexibility.
Certain countries have legal or economic restrictions
on the ability of subsidiaries to transfer funds to the
Group in the form of cash dividends, loans or advances,
but these restrictions do not have an impact on the abil-
ity of the Group to meet its cash obligations.
Management monitors the Group’s net debt or liquid-
ity position through rolling forecasts on the basis of
expected cash flows.
Novartis has two US commercial paper programs
under which it can issue up to USD 9.0 billion in the
aggregate of unsecured commercial paper notes.
Novartis also has one Japanese commercial paper pro-
gram under which it can issue up to JPY 150 billion
(approximately USD1.1 billion) of unsecured commercial
paper notes. Commercial paper notes totaling USD2.8
billion under these three programs were outstanding as
per December 31, 2022 (2021: USD0.9 billion). Novartis
further has a committed credit facility of USD6.0 billion,
which was extended in September 2022. This credit
facility is provided by a syndicate of banks and is intended
to be used as a backstop for the US commercial paper
programs. The facility matures in September 2025 and
was undrawn as per December 31, 2022, and December
31, 2021.

Notes to the Novartis Groupconsolidatedfinancial statements
F-71
The following table sets forth how management monitors net debt or liquidity based on details of the remaining
contractual maturities of current financial assets and liabilities, excluding trade receivables and payables as well
as liabilities for contingent consideration at December 31, 2022, and December 31, 2021:
2022
Due later than
Due later than
Due later than
one month
three months
one year
Due within
but less than
but less than
but less than
Due after
(USD millions) one month
three months
one year
five years
five years
Total
Current assets
Marketable securities, time deposits and short-term
investments with original maturity more than days
and accrued interest
Commodities
Derivative financial instruments
Cash and cash equivalents
Total current financial assets
Non-current liabilities
Financial debt
–
–
–
Financial debt – undiscounted
– 9 002
– 11 394
– 20 396
Total non-current financial debt
–
–
–
Current liabilities
Financial debt –
–
–
–
Financial debt – undiscounted – 3 215
– 146
– 2 517
– 5 878
Derivative financial instruments –
–
–
–
Total current financial debt –
–
–
–
Net debt
–
–
–
–
2021
Due later than
Due later than
Due later than
one month
three months
one year
Due within
but less than
but less than
but less than
Due after
(USD millions) one month
three months
one year
five years
five years
Total
Current assets
Marketable securities, time deposits and short-term
investments with original maturity more than days
and accrued interest
Commodities
Derivative financial instruments
Cash and cash equivalents
Total current financial assets
Non-current liabilities
Financial debt
–
–
–
Financial debt – undiscounted
– 8 490
– 14 587
– 23 077
Total non-current financial debt
–
–
–
Current liabilities
Financial debt –
–
–
–
Financial debt – undiscounted – 2 780
– 521
– 2 928
– 6 229
Derivative financial instruments –
–
–
–
Total current financial debt –
–
–
–
Net debt
–
–
–
–
The carrying amounts of financial liabilities included in the above analysis are not materially dierent to the con-
tractual amounts due on maturity. The positive and negative fair values on derivative financial instruments repre-
sent the net contractual amounts to be exchanged at maturity.

Notes to the Novartis Groupconsolidatedfinancial statements
F-72
The Group’s contractual undiscounted potential cash flows from derivative financial instruments to be settled
on a gross basis are as follows:
2022
Due later than
Due later than
one month
three months
Due within
but less than
but less than
(USD millions) one month
three months
one year Total
Derivative financial instruments and accrued interest on derivative
financial instruments
Potential outflows in various currencies – from financial derivative liabilities –
–
–
–
Potential inflows in various currencies – from financial derivative assets
2021
Due later than
Due later than
one month
three months
Due within
but less than
but less than
(USD millions) one month
three months
one year Total
Derivative financial instruments and accrued interest on derivative
financial instruments
Potential outflows in various currencies – from financial derivative liabilities –
–
–
–
Potential inflows in various currencies – from financial derivative assets
Other contractual liabilities that are not part of management’s monitoring of the net debt or liquidity consist of the
following items:
2022
Due later than
Due later than
three months
one year
Due within
but less than
but less than
Due after
(USD millions) three months
one year
five years
five years
Total
Contractual interest on non-current liabilities –
–
–
–
–
Lease liabilities
–
–
–
–
–
Trade payables –
–
–
Contingent consideration liabilities –
–
–
–
–
1
Note 10 provides additional disclosures related to lease liabilities.
2021
Due later than
Due later than
three months
one year
Due within
but less than
but less than
Due after
(USD millions) three months
one year
five years
five years
Total
Contractual interest on non-current liabilities –
–
–
–
–
Lease liabilities
–
–
–
–
–
Trade payables –
–
–
Commitment for repurchase of own shares –
–
Contingent consideration liabilities –
–
–
–
–
1
Note 10 provides additional disclosures related to lease liabilities.
Capital risk management
Novartis strives to maintain a strong credit rating. In man-
aging its capital, Novartis focuses on maintaining a
strong balance sheet. As of December 31, 2022, Moody’s
Investors Service rated the Company A1 for long-term
maturities and P-1 for short-term maturities, and S&P
Global Ratings rated the Company AA- for long-term
maturities and A-1+ for short-term maturities.
Sensitivity analysis
The Group uses sensitivity analysis disclosures to pro-
vide quantitative information about market risks to which
it is exposed.
The sensitivity analysis disclosures are in line with
the Group’s financial risk management policy, and are
based on a one-parameter risk model that considers a
one-factor linear relationship between risk factors and

Notes to the Novartis Groupconsolidatedfinancial statements
F-73
exposures. They consider aggregated risk exposures
arising from the most significant risk factors (currency
risk, interest rate risk and equity price risk) and include
all financial assets and financial liabilities as set forth in
the table on page F-64.
The disclosures below illustrate the potential impact
on the Group’s consolidated financial statements as a
result of hypothetical market movements in foreign cur-
rency exchange rates, interest rates and equity prices.
The range of variables chosen reflects management’s
view of changes that are reasonably possible over a one-
year period.
Foreign currency exchange rate sensitivity
The Group uses the US dollar as its reporting currency.
As a result, the Group is exposed to foreign currency
exchange movements, primarily in European, Japanese
and emerging market currencies, as well as in the Swiss
franc. A strengthening (weakening) of the US dollar
against these currencies as of December 31, 2022 and
2021 would have aected the measurement of financial
instruments denominated in these foreign currencies.
This analysis assumes that all other variables, in partic-
ular interest rates, remain constant. A hypothetical 5%
increase or decrease in the foreign currency exchange
rates against the US dollar would have impacted the
Group’s consolidated income statement as presented
below:
(USD millions)
increase in foreign currency exchange rates
against USD –
decrease in foreign currency exchange rates
against USD
–
As of December 31, 2022, the Group designated EUR 1.8
billion (December 31, 2021: EUR 1.8 billion) of its long-
term euro-denominated straight bonds as hedges of the
translation risk arising on certain net investments in for-
eign operations with euro functional currency. This anal-
ysis assumes that all other variables, in particular inter-
est rates, remain constant. A hypothetical 5% increase,
or decrease, in the foreign currency exchange rates
against the US dollar, without considering the translation
eect of these net investments, would have impacted
the Group’s consolidated equity as presented below:
(USD millions)
increase in foreign currency exchange rates
against USD
decrease in foreign currency exchange rates
against USD –
–
Interest rate sensitivity
Our portfolio of fixed-income instruments as of Decem-
ber 31, 2022, was mainly composed of time deposits and
debt securities.
Novartis uses duration models to approximate the
possible change in the value of fixed-income instru
-
ments. Based on these models, management believes
that a 100-basis point change in interest is deemed a
reasonable possible change over a one-year period.
Based on exposures in 2022 and 2021, a hypotheti-
cal 100-basis point increase (decrease) in interest rates
would not have resulted in a significant increase
(decrease) in the fair values of the fixed-income instru-
ments. In addition, a hypothetical 100-basis point
increase (decrease) in interest rates would not have
resulted in a material increase (decrease) of cash flows
attributable to such fixed-income instruments.
The vast majority of our outstanding financial debts
are straight bonds with fixed interest rates and are there-
fore not aected by movements in interest rates.
Equity price sensitivity
Fund investments and equity securities held by the
Novartis Venture Fund are valued at fair value through
profit and loss. Equity securities held as strategic invest-
ments, typically held outside the Novartis Venture Fund,
are generally designated at date of acquisition as finan-
cial assets valued at fair value through other compre-
hensive income with no subsequent recycling through
profit and loss.
The fair value of these fund investments and equity
securities was USD 1.6 billion as of December 31, 2022
(December 31, 2021: USD 2.2 billion). The fair values of
these investments are impacted by the volatility of the
stock market, valuation parameters applied (for non-
listed equities) and changes in general economic factors.
This analysis assumes that all other variables, in partic-
ular interest rates, remain constant. A hypothetical
increase or decrease of 15% in the risk factors would
have impacted the Group’s consolidated income state-
ment as presented below:
(USD millions)
increase in equity prices
decrease in equity prices –
–
A hypothetical increase or decrease of 15% in the risk
factors would have impacted the Group’s consolidated
equity as presented below:
(USD millions)
increase in equity prices
decrease in equity prices –
–

Notes to the Novartis Groupconsolidatedfinancial statements
F-74
30. Events subsequent to the December 31, 2022,
consolidated balance sheet date
South Korea investigation – Concluded matter
In January 2023, the Supreme Court dismissed the
appeal by the Seoul Western District Prosecutor on the
criminal investigation on, among other things, allegations
that Novartis Korea utilized medical journals to provide
inappropriate economic benefits to healthcare profes-
sionals (HCPs). This matter is now concluded. For addi-
tional information see Note 20.
Dividend proposal for 2022 and approval ofthe
Group’s 2022 consolidated financial statements
On January 31, 2023, the Novartis AG Board of Direc-
tors proposed the acceptance of the 2022 consolidated
financial statements of the Novartis Group for approval
by the Annual General Meeting on March 7, 2023. Fur-
thermore, also on January 31, 2023, the Board proposed
a dividend of CHF 3.20 per share to be approved at the
Annual General Meeting on March 7, 2023. If approved,
total dividend payments would amount to approximately
USD 7.3 billion (2021: USD7.5 billion), using the CHF/USD
December 31, 2022, exchange rate.

Notes to the Novartis Groupconsolidatedfinancial statements
F-75
31. Principal Group subsidiaries
andassociatedcompanies
The following table lists the principal subsidiaries controlled by Novartis, associated companies in which Novartis
is deemed to have significant influence, and foundations required to be consolidated under IFRS. It includes all sub-
sidiaries, associated companies and consolidated foundations with total assets or net sales to third parties in excess
of USD25million. The equity interest percentage shown in the table also represents the share in voting rights in
those entities.
Share
Equity
As at December 31, 2022 capital
1
interest
Algeria
Société par actions SANDOZ, Algiers DZD 650.0 m 100%
Argentina
Novartis Argentina S.A., Buenos Aires ARS 906.1 m 100%
Australia
Novartis Australia Pty Ltd, Macquarie Park, NSW AUD 2 100%
Novartis Pharmaceuticals
Australia Pty Ltd, Macquarie Park, NSW AUD 3.8 m 100%
Sandoz Pty Ltd, Macquarie Park, NSW AUD 11.6 m 100%
Austria
Novartis Austria GmbH, Vienna EUR 1.0 m 100%
Novartis Pharma GmbH, Vienna EUR 1.1 m 100%
Sandoz GmbH, Kundl EUR 32.7 m 100%
EBEWE Pharma Ges.m.b.H Nfg. KG, Unterach am Attersee EUR 1.0 m 100%
Bangladesh
Novartis (Bangladesh) Limited, Gazipur BDT 162.5 m 60%
Belgium
Novartis Pharma NV, Vilvoorde EUR 72.1 m 100%
Sandoz NV, Vilvoorde EUR 19.2 m 100%
Alcon – Couvreur NV, Puurs EUR 110.6 m 100%
Bermuda
Novartis Investment Ltd., Hamilton
2
USD 12 000 100%
Novartis Securities Investment Ltd., Hamilton CHF 30 000 100%
Novartis Finance Services Ltd., Hamilton CHF 20 000 100%
Triangle International Reinsurance Limited, Hamilton CHF 1.0 m 100%
Trinity River Insurance Co Ltd., Hamilton USD 370 000 100%
Brazil
Novartis Biociências S.A., São Paulo BRL 507.1 m 100%
Sandoz do Brasil Indústria Farmacêutica Ltda., Cambé, PR BRL 190.0 m 100%
Canada
Novartis Pharmaceuticals Canada Inc., Dorval, Quebec CAD 1.2 m 100%
Sandoz Canada Inc., Boucherville, Quebec CAD 80.8 m 100%
Chile
Novartis Chile S.A., Santiago de Chile CLP 2.0 bn 100%
China
Beijing Novartis Pharma Co., Ltd., Beijing USD 30.0 m 100%
Novartis Pharmaceuticals (HK) Limited, Hong Kong HKD 200 100%
China Novartis Institutes for
BioMedical Research Co., Ltd., Shanghai USD 320.0 m 100%
Suzhou Novartis Technical
Development Co., Ltd., Changshu USD 12.0 m 100%
Shanghai Novartis Trading Ltd., Shanghai USD 3.2 m 100%
Sandoz (China) Pharmaceutical
Co., Ltd., Zhongshan USD 57.6 m 100%
Colombia
Novartis de Colombia S.A., Santafé de Bogotá COP 7.9 bn 100%
Croatia
Sandoz d.o.o. farmaceutska industrija, Zagreb HRK 25.6 m 100%
Czech Republic
Novartis s.r.o., Prague CZK 51.5 m 100%
Sandoz s.r.o., Prague CZK 44.7 m 100%
Denmark
Novartis Healthcare A/S, Copenhagen DKK 14.0 m 100%
Sandoz A/S, Copenhagen DKK 12.0 m 100%
Ecuador
Novartis Ecuador S.A., Quito USD 4.0 m 100%
Egypt
Novartis Pharma S.A.E., Cairo EGP 1.3 bn 99.96%
Sandoz Egypt Pharma S.A.E., New Cairo City EGP 250 000 100%
Finland
Novartis Finland Oy, Espoo EUR 459 000 100%
Share
Equity
As at December 31, 2022 capital
1
interest
France
Novartis Groupe France S.A., Rueil-Malmaison EUR 903.0 m 100%
Novartis Pharma S.A.S., Rueil-Malmaison EUR 43.4 m 100%
Novartis Gene Therapies France SAS, Rueil-Malmaison EUR 10 000 100%
Advanced Accelerator Applications S.A., Rueil-Malmaison EUR 9.6 m 99.23%
Advanced Accelerator Applications
Molecular Imaging France, Saint-Genis-Pouilly EUR 7.5 m 99.23%
CELLforCURE, Les Ulis EUR 4.2 m 100%
Sandoz S.A.S., Levallois-Perret EUR 5.4 m 100%
Germany
Novartis Deutschland GmbH, Nuremberg EUR 155.5 m 100%
Novartis Business Services GmbH, Wehr EUR 25 000 100%
Novartis Pharma GmbH, Nuremberg EUR 25.6 m 100%
Novartis Pharma Produktions GmbH, Wehr EUR 2.0 m 100%
Sandoz International GmbH, Holzkirchen EUR 100 000 100%
1 A Pharma GmbH, Holzkirchen EUR 26 000 100%
HEXAL AG, Holzkirchen EUR 93.7 m 100%
Salutas Pharma GmbH, Barleben EUR 42.1 m 100%
Aeropharm GmbH, Rudolstadt EUR 26 000 100%
Greece
Novartis (Hellas) S.A.C.I., Metamorphosis / Athens EUR 233.9 m 100%
Hungary
Novartis Hungary Healthcare Limited Liability
Company, Budapest HUF 545.6 m 100%
Sandoz Hungary Limited Liability Company, Budapest HUF 883.0 m 100%
India
Novartis India Limited, Mumbai INR 123.5 m 70.68%
Novartis Healthcare Private Limited, Mumbai INR 60.0 m 100%
Sandoz Private Limited, Mumbai INR 32.0 m 100%
Indonesia
PT. Novartis Indonesia, Jakarta IDR 7.7 bn 100%
Ireland
Novartis Ireland Limited, Dublin EUR 25 000 100%
Novartis Integrated Services Limited, Cork City EUR 100 100%
Novartis Gene Therapies EU Limited, Dublin EUR 100 100%
Israel
Novartis Israel Ltd., Tel Aviv ILS 1 000 100%
Italy
Novartis Farma S.p.A., Milan EUR 18.2 m 100%
Advanced Accelerator Applications (Italy) S.r.l., Pozzilli EUR 119 000 99.23%
Sandoz S.p.A., Origgio EUR 1.7 m 100%
Japan
Novartis Pharma K.K., Tokyo JPY 100.0 m 100%
Ciba-Geigy Japan Limited, Tokyo JPY 100.0 m 100%
Sandoz K.K., Tokyo JPY 100.0 m 100%
Latvia
Novartis Baltics SIA, Riga EUR 3.0 m 100%
Luxembourg
Novartis Investments S.à r.l., Luxembourg City
2
USD 100.0 m 100%
Novartis Finance S.A., Luxembourg City USD 100 000 100%
Malaysia
Novartis Corporation (Malaysia) Sdn. Bhd., Petaling Jaya MYR 3.3 m 100%
Mexico
Novartis Farmacéutica, S.A. de C.V., Mexico City MXN 205.0 m 100%
Sandoz, S.A. de C.V., Mexico City MXN 468.2 m 100%
Morocco
Novartis Pharma Maroc SA, Casablanca MAD 80.0 m 100%
Netherlands
Novartis Netherlands B.V., Amsterdam EUR 1.4 m 100%
Novartis Pharma B.V., Amsterdam EUR 4.5 m 100%
IDB Holland BV, Baarle-Nassau EUR 18 000 99.23%
Sandoz B.V., Almere EUR 907 560 100%
New Zealand
Novartis New Zealand Ltd, Auckland NZD 820 000 100%

Notes to the Novartis Groupconsolidatedfinancial statements
F-76
Share
Equity
As at December 31, 2022 capital
1
interest
Norway
Novartis Norge AS, Oslo NOK 1.5 m 100%
Pakistan
Novartis Pharma (Pakistan) Limited, Karachi PKR 6.7 bn 99.99%
Panama
Novartis Pharma (Logistics), Inc., Panama City USD 10 000 100%
Philippines
Novartis Healthcare Philippines, Inc., Makati City PHP 298.8 m 100%
Sandoz Philippines Corporation, Makati City PHP 30.0 m 100%
Poland
Novartis Poland Sp. z o.o., Warsaw PLN 44.2 m 100%
Sandoz Polska Sp. z o.o., Warsaw PLN 25.6 m 100%
Lek S.A., Strykow PLN 11.4 m 100%
Portugal
Novartis Portugal, S.G.P.S., Lda., Porto Salvo EUR 500 000 100%
Novartis Farma – Produtos Farmacêuticos, S.A., Porto Salvo EUR 2.4 m 100%
Sandoz Farmacêutica, Lda., Porto Salvo EUR 499 900 100%
Romania
Novartis Pharma Services Romania S.R.L., Bucharest RON 3.0 m 100%
Sandoz S.R.L., Targu-Mures RON 119.5 m 100%
Russian Federation
Novartis Pharma LLC, Moscow RUB 20.0 m 100%
Novartis Neva LLC, St. Petersburg RUB 500.0 m 100%
JSC Sandoz, Moscow RUB 57.4 m 100%
Saudi Arabia
Novartis Saudi Ltd., Riyadh SAR 30.0 m 100%
Singapore
Novartis (Singapore) Pte Ltd., Singapore SGD 100 000 100%
Novartis Singapore Pharmaceutical
Manufacturing Pte Ltd, Singapore SGD 45.0 m 100%
Novartis Asia Pacific Pharmaceuticals
Pte Ltd, Singapore SGD 39.0 m 100%
Slovakia
Novartis Slovakia s.r.o., Bratislava EUR 2.0 m 100%
Slovenia
Lek Pharmaceuticals d.d., Ljubljana EUR 48.4 m 100%
Sandoz Pharmaceuticals d.d., Ljubljana EUR 1.5 m 100%
South Africa
Novartis South Africa (Pty) Ltd, Midrand ZAR 86.3 m 100%
Sandoz South Africa (Pty) Ltd, Midrand ZAR 3.0 m 100%
South Korea
Novartis Korea Ltd., Seoul KRW 24.5 bn 100%
Spain
Novartis Farmacéutica, S.A., Barcelona EUR 63.0 m 100%
Advanced Accelerator Applications
Iberica, S.L.U., Esplugues de Llobregat EUR 22.6 m 99.23%
Sandoz Farmacéutica S.A., Madrid EUR 270 450 100%
Sandoz Industrial Products
S.A., Les Franqueses del Vallés / Barcelona EUR 9.3 m 100%
Abadia Retuerta S.A., Sardón de Duero / Valladolid EUR 6.0 m 100%
Sweden
Novartis Sverige AB, Stockholm SEK 5.0 m 100%
Switzerland
Novartis International AG, Basel CHF 10.0 m 100%
Novartis Holding AG, Basel
2
CHF 100.2 m 100%
Novartis International Pharmaceutical Investment AG, Basel CHF 100 000 100%
Novartis Bioventures AG, Basel CHF 100 000 100%
Novartis Forschungsstiftung, Basel
3
-- -- --
Novartis Stiftung für Kaderausbildung, Basel
3
-- -- --
Novartis-Mitarbeiterbeteiligungsstiftung, Basel
3
-- -- --
Novartis Stiftung für Mensch und Umwelt, Basel
3
-- -- --
Stiftung der Novartis AG für Erziehung,
Ausbildung und Bildung, Basel
3
-- -- --
Novartis Overseas Investments AG, Basel CHF 1.0 m 100%
Japat AG, Basel CHF 50 000 100%
Novartis Pharma AG, Basel
2
CHF 350.0 m 100%
Novartis Pharma Services AG, Basel CHF 20.0 m 100%
Novartis Pharma Schweizerhalle AG, Muttenz CHF 18.9 m 100%
Novartis Pharma Stein AG, Stein CHF 251 000 100%
Novartis Pharma Schweiz AG, Risch CHF 5.0 m 100%
Cellerys AG, Schlieren CHF 129 630 20%
Arctos Medical AG, Bern CHF 360 020 100%
Novartis Innovative Therapies AG, Risch CHF 100 000 100%
Advanced Accelerator Applications International SA, Geneva CHF 9.3 m 99.23%
Sandoz AG, Basel
2
CHF 5.0 m 100%
Sandoz Pharmaceuticals AG, Risch CHF 100 000 100%
Share
Equity
As at December 31, 2022 capital
1
interest
Taiwan
Novartis (Taiwan) Co., Ltd., Taipei TWD 170.0 m 100%
Thailand
Novartis (Thailand) Limited, Bangkok THB 302.0 m 100%
Turkey
Novartis Saglik, Gida ve Tarim Ürünleri Sanayi
ve Ticaret A.S., Istanbul TRY 448.0 m 100%
Farmanova Saglik Hizmetleri Ltd. Sti., Istanbul TRY 6.7 m 100%
Sandoz Ilaç Sanayi ve Ticaret A.S., Istanbul TRY 880.0 m 99.99%
Sandoz Grup Saglik Ürünleri
Ilaçlari Sanayi ve Ticaret A.S., Gebze – Kocaeli TRY 96.0 m 100%
Ukraine
Sandoz Ukraine LLC, Kyiv UAH 8.0 m 100%
United Arab Emirates
Novartis Middle East FZE, Dubai AED 7.0 m 100%
United Kingdom
Novartis UK Limited, London GBP 25.5 m 100%
Novartis Pharmaceuticals UK Limited, London GBP 5.4 m 100%
Novartis Grimsby Limited, London GBP 250.0 m 100%
Advanced Accelerator Applications (UK & Ireland), London GBP 100 99.23%
Neutec Pharma Limited, London GBP 7.7 m 100%
Gyroscope Therapeutics Limited, London GBP 1 492 100%
Sandoz Limited, Frimley / Camberley GBP 2.0 m 100%
United States of America
Novartis Corporation, East Hanover, NJ USD 72.2 m 100%
Novartis Finance Corporation, East Hanover, NJ
2
USD 1 000 100%
Novartis Capital Corporation, East Hanover, NJ USD 1 100%
Novartis Services, Inc., East Hanover, NJ USD 1 100%
Novartis US Foundation, East Hanover, NJ
3
-- -- --
Novartis Pharmaceuticals Corporation, East Hanover, NJ
2
USD 650 100%
Advanced Accelerator Applications USA, Inc., Millburn, NJ USD 1 99.23%
Novartis Gene Therapies, Inc., Bannockburn, IL USD 1 100%
Novartis Technology LLC, East Hanover, NJ -- -- --
Novartis Institutes for BioMedical
Research, Inc., Cambridge, MA USD 1 100%
Cadent Therapeutics, Cambridge, MA USD 0.1 100%
Endocyte, Inc., East Hanover, NJ USD 1 100%
Navigate BioPharma Services, Inc., Carlsbad, CA USD 1 100%
The Medicines Company, East Hanover, NJ USD 1 000 100%
Sandoz Inc., Princeton, NJ USD 25 000 100%
Oriel Therapeutics, Inc., Durham, NC USD 50.0 m 100%
Fougera Pharmaceuticals Inc., Melville, NY USD 1 100%
Eon Labs, Inc., Princeton, NJ USD 1 100%
Venezuela
Novartis de Venezuela, S.A., Caracas VES 0 100%
Vietnam
Novartis Vietnam Company Limited, Ho Chi Minh City VND 70 bn 100%
In addition, the Group is represented by subsidiaries and associated companies with
total assets or net sales to third parties below USD 25 million in the following countries:
Bosnia and Herzegovina, Bulgaria, Cameroon, Dominican Republic, Ghana, Guatemala,
Ivory Coast, Kazakhstan, Kenya, Kuwait, North Macedonia, Nigeria, Peru, Senegal and
Uruguay.
1
Share capital may not reflect the taxable share capital and does not include any
paid-in surplus.
2
Significant subsidiary under SEC Regulation SX Rule 1-02(w)
3
Fully consolidated Foundation
m = million; bn = billion

Statutory Auditor’s Report
F-77
Statutory Auditor’s Report
to the General Meeting of NovartisAG
Basel
Report on the Audit of the
Consolidated Financial Statements
Opinion
We have audited the consolidated financial statements
of Novartis AG and its subsidiaries (“the Group”), which
comprise the consolidated balance sheet as at Decem-
ber 31, 2022, the consolidated income statement, con-
solidated statement of comprehensive income, consol-
idated statement of changes in equity and consolidated
statement of cash flows for the year then ended, and
notes to the consolidated financial statements, including
a summary of significant accounting policies.
In our opinion, the consolidated financial statements
(pages F-1 to F-76) give a true and fair view of the con-
solidated financial position of the Group as at December
31, 2022, and its consolidated financial performance and
its consolidated cash flows for the year then ended in
accordance with International Financial Reporting Stan-
dards (IFRS) and comply with Swiss law.
Basis for opinion
We conducted our audit in accordance with Swiss law,
International Standards on Auditing (ISAs) and Swiss
Standards on Auditing (SACH). Our responsibilities
under those provisions and standards are further
described in the “Auditor’s Responsibilities for the Audit
of the Consolidated Financial Statements” section of our
report. We are independent of the Group in accordance
with the provisions of Swiss law, together with the
requirements of the Swiss audit profession, as well as
the International Ethics Standards Board for Accoun-
tants’ International Code of Ethics for Professional
Accountants (including International Independence
Standards) (IESBA Code), and we have fulfilled our other
ethical responsibilities in accordance with these require-
ments.
We believe that the audit evidence we have obtained
is sucient and appropriate to provide a basis for our
opinion.
Key Audit Matters
• Assessment of the recoverable amount for the Leqvio
and Xiidra intangible assets
• Provisions for deductions from revenue related to
Innovative Medicines US Managed Care, Medicare
PartD and Medicaid rebate programs
Key audit matters are those matters that, in our profes-
sional judgment, were of most significance in our audit
of the consolidated financial statements of the current
period. These matters were addressed in the context of
our audit of the consolidated financial statements as a
whole, and in forming our opinion thereon, and we do not
provide a separate opinion on these matters.
Assessment of the recoverable amount for the
Leqvio and Xiidra intangible assets
Key Audit Matter
As discussed in Note 1 to the consolidated financial state-
ments, the Group determined the recoverable amount
of the intangible assets other than goodwill based on the
fair value less costs of disposal method for which no
directly observable market inputs were available. As dis-
cussed in Note 11, the Group has intangible assets in its
Innovative Medicines Division other than goodwill total-
ing USD 29’826 million as at December 31, 2022, a por-
tion of which related to the currently marketed products
Leqvio and Xiidra.
We identified the assessment of the recoverable
amount, specifically the sales forecasts of the Leqvio
and Xiidra intangible assets, as a Key Audit Matter. Sig-
nificant auditor judgment and subjectivity was required
to assess the sales forecasts assumptions which were
a significant input in the determination of the recover-
able amount of these intangible assets.
Our response
The following are the primary procedures we performed
to address this Key Audit Matter:
• We evaluated the design and tested the operating
eectiveness of a certain internal control related to the
Group’s intangible asset impairment process for Leqvio
and Xiidra, including the development of the sales fore-
casts;
• We evaluated the reasonableness of management’s
sales forecasts for Leqvio and Xiidra by (1) comparing
certain underlying assumptions to company-specific
operational information and management’s communi-
cations to the board of directors, (2) comparing the
most recent sales performance to previous drug
launches, and (3) comparing certain underlying
assumptions to available external market and industry
data; and
• We assessed management’s ability to accurately fore-
cast sales by comparing historical sales forecasts for
Leqvio and Xiidra to actual results.
For further information on the assessment of the recov-
erable amount for the Leqvio and Xiidra intangible assets
refer to the following:
Page F-6 (Note 1 Significant accounting policies), Page
F-17 (Note 3 Segmentation of key figures 2022, 2021 and
2020) and Page F-33 (Note 11 Goodwill and intangible
assets)

Statutory Auditor’s Report
F-78
Provisions for deductions from revenue related to
Innovative Medicines US Managed Care, Medicare
Part D and Medicaid rebate programs
Key Audit Matter
As discussed in Note 1 to the consolidated financial state-
ments, the Group records provisions for estimated
rebates as a deduction from revenue when the related
revenue is recognized. Rebates involve the use of
assumptions and judgements in the determination of the
provision rates at the time revenues are recorded. Pro-
vision rates are influenced by the terms and conditions
in the individual agreements, historical experience, prod-
uct sales and growth rate, population growth, product
pricing, the mix of contracts and products, the level of
inventory in the distribution channel, regulations, con-
tracts, and channels and payers. As discussed in Note
22, provisions for deductions from revenue totaled USD
6’732 million as at December 31, 2022, a portion of which
related to Innovative Medicines US Managed Care, Medi-
care Part D and Medicaid rebate programs (hereafter
“IM US rebates”).
We identified the estimation of the IM US rebates pro-
visions, specifically the provision rebate rates, as a Key
Audit Matter. The evaluation of the provision rebate rates
required a high degree of subjective auditor judgment as
it involved estimating the portion of the Group’s revenue
which will ultimately be subject to a related rebate.
Our response
The following are the primary audit procedures we per-
formed to address this Key Audit Matter:
• We evaluated the design and tested the operating
eectiveness of certain internal controls over the
Group’s IM US rebates process related to the develop-
ment of the provision rebate rates;
• We developed our own independent expectation of the
IM US rebates provisions, by using internal information,
including historical experience and trend analysis of
actual rebate claims paid, and comparing it to manage-
ment’s actual recorded balances;
• For a sample of actual rebate claims processed by the
Group, we evaluated the claims against the contractual
and mandated terms of the rebate arrangements; and
• We assessed management’s ability to accurately esti-
mate the IM US rebates provisions by comparing his-
torically recorded provisions to the actual amount that
was ultimately paid by the Group.
For further information on the provisions for deductions
from revenue related to Innovative Medicines US Man-
aged Care, Medicare Part D and Medicaid rebate pro-
grams refer to the following:
Page F-6 (Note 1 Significant accounting policies), Page
F-17 (Note 3 Segmentation of key figures 2022, 2021 and
2020), Page F-38 (Note 15 Trade receivables) and Page
F-48 (Note 22 Provisions and other current liabilities)
Other Matter
The consolidated financial statements of the Group for
the year ended December 31, 2021 were audited by
another auditor who expressed an unmodified opinion
on those statements on February 1, 2022.
Other Information in the
AnnualReport
The Board of Directors is responsible for the other infor-
mation in the Annual Report. The other information com-
prises the information included in the Annual Report, but
does not include the consolidated financial statements,
the stand-alone financial statements of the company, the
compensation report and our auditor’s reports thereon.
Our opinion on the consolidated financial statements
does not cover the other information in the Annual Report
and we do not express any form of assurance conclu-
sion thereon.
In connection with our audit of the consolidated finan-
cial statements, our responsibility is to read the other
information in the Annual Report and, in doing so, con-
sider whether the other information is materially incon-
sistent with the consolidated financial statements or our
knowledge obtained in the audit or otherwise appears
to be materially misstated.
If, based on the work we have performed, we con-
clude that there is a material misstatement of this other
information, we are required to report that fact. We have
nothing to report in this regard.
Board of Directors’ Responsibilities
for the Consolidated Financial
Statements
The Board of Directors is responsible for the prepara-
tion of the consolidated financial statements that give a
true and fair view in accordance with IFRS and the pro-
visions of Swiss law, and for such internal control as the
Board of Directors determines is necessary to enable
the preparation of consolidated financial statements that
are free from material misstatement, whether due to
fraud or error.
In preparing the consolidated financial statements,
the Board of Directors is responsible for assessing the
Group’s ability to continue as a going concern, disclos-
ing, as applicable, matters related to going concern and
using the going concern basis of accounting unless the
Board of Directors either intends to liquidate the Group
or to cease operations, or has no realistic alternative but
to do so.
Auditor’s Responsibilities for the
Audit of the Consolidated Financial
Statements
Our objectives are to obtain reasonable assurance about
whether the consolidated financial statements as a
whole are free from material misstatement, whether due
to fraud or error, and to issue an auditor’s report that
includes our opinion. Reasonable assurance is a high
level of assurance, but is not a guarantee that an audit
conducted in accordance with Swiss law, ISAs and

Statutory Auditor’s Report
F-79
SACH will always detect a material misstatement when
it exists. Misstatements can arise from fraud or error and
are considered material if, individually or in the aggre-
gate, they could reasonably be expected to influence the
economic decisions of users taken on the basis of these
consolidated financial statements.
As part of an audit in accordance with Swiss law, ISAs
and SACH, we exercise professional judgment and
maintain professional skepticism throughout the audit.
We also:
• Identify and assess the risks of material misstatement
of the consolidated financial statements, whether due
to fraud or error, design and perform audit procedures
responsive to those risks, and obtain audit evidence
that is sucient and appropriate to provide a basis for
our opinion. The risk of not detecting a material mis-
statement resulting from fraud is higher than for one
resulting from error, as fraud may involve collusion,
forgery, intentional omissions, misrepresentations, or
the override of internal control.
• Obtain an understanding of internal control relevant to
the audit in order to design audit procedures that are
appropriate in the circumstances, but not for the pur-
pose of expressing an opinion on the eectiveness of
the Group’s internal control.
• Evaluate the appropriateness of accounting policies
used and the reasonableness of accounting estimates
and related disclosures made.
• Conclude on the appropriateness of the Board of
Directors’ use of the going concern basis of account-
ing and, based on the audit evidence obtained, whether
a material uncertainty exists related to events or con-
ditions that may cast significant doubt on the Group’s
ability to continue as a going concern. If we conclude
that a material uncertainty exists, we are required to
draw attention in our auditor’s report to the related dis-
closures in the consolidated financial statements or, if
such disclosures are inadequate, to modify our opin-
ion. Our conclusions are based on the audit evidence
obtained up to the date of our auditor’s report. How-
ever, future events or conditions may cause the Group
to cease to continue as a going concern.
• Evaluate the overall presentation, structure and con-
tent of the consolidated financial statements, including
the disclosures, and whether the consolidated finan-
cial statements represent the underlying transactions
and events in a manner that achieves fair presentation.
• Obtain sucient appropriate audit evidence regarding
the financial information of the entities or business
activities within the Group to express an opinion on the
consolidated financial statements. We are responsible
for the direction, supervision and performance of the
Group audit. We remain solely responsible for our audit
opinion.
We communicate with the Board of Directors, primarily
through the Audit and Compliance Committee, regard-
ing, among other matters, the planned scope and timing
of the audit and significant audit findings, including any
significant deficiencies in internal control that we iden-
tify during our audit.
We also provide the Board of Directors with a state-
ment that we have complied with relevant ethical require-
ments regarding independence, and communicate with
them all relationships and other matters that may rea-
sonably be thought to bear on our independence, and
where applicable, actions taken to eliminate threats or
safeguards applied.
From the matters communicated with the Board of
Directors, we determine those matters that were of most
significance in the audit of the consolidated financial
statements of the current period and are therefore the
key audit matters. We describe these matters in our audi-
tor’s report, unless law or regulation precludes public
disclosure about the matter or when, in extremely rare
circumstances, we determine that a matter should not
be communicated in our report because the adverse
consequences of doing so would reasonably be expected
to outweigh the public interest benefits of such commu-
nication.
Report on Other Legal and
Regulatory Requirements
In accordance with article 728a para. 1 item 3 CO and
PSCH 890, we confirm that an internal control system
exists, which has been designed for the preparation of
consolidated financial statements according to the
instructions of the Board of Directors.
We recommend that the consolidated financial state-
ments submitted to you be approved.
KPMG AG
Richard Broadbelt Sara Burke
Licensed Audit Expert
Auditor in charge
Basel, January 31, 2023

Financial statements of Novartis AG
A-1
Financial statements of Novartis AG
Income statements
(For the years ended December 31, 2022 and 2021)
(CHF millions) Note
Income from investment in Group subsidiaries
License income
Other income
Total income
Amortization of goodwill
–
–
Impairment of investment in Group subsidiaries
–
General and administrative expenses
–
–
Total expenses
–
–
Operating income
Financial income
Financial expenses
–
–
Extraordinary expenses
–
Income before taxes
Direct taxes
–
–
Net income of the year
The accompanying Notes form an integral part of these financial statements.

Financial statements of Novartis AG
A-2
Balance sheets
(At December 31, 2022 and 2021)
(CHF millions) Note
Assets
Current assets
Cash and cash equivalents
Interest-bearing current receivables
Group subsidiaries
Other current receivables
Group subsidiaries
Total current assets
Non-current assets
Financial assets
Group subsidiaries
Investments
Group subsidiaries
Goodwill
Total non-current assets
Total assets
Liabilities and equity
Current liabilities
Interest-bearing current liabilities
Group subsidiaries
Other current liabilities
Group subsidiaries
Third parties
Accrued expenses
Total current liabilities
Non-current liabilities
Interest-bearing non-current liabilities
Bonds
Non-current provisions
Total non-current liabilities
Total liabilities
Equity
Share capital
Legal reserves
General legal reserve
Legal reserve for treasury shares held by subsidiaries
Total legal reserves
Free reserves
Retained earnings
Net income of the year
Retained earnings available for distribution at the end of the year
Total unappropriated earnings and free reserves
Treasury shares held by Novartis AG
–
–
Total equity
Total liabilities and equity
The accompanying Notes form an integral part of these financial statements.

Notes to the financial statements ofNovartisAG
A-3
Notes to the financial statements
ofNovartisAG
1. Introduction
The financial statements of Novartis AG, with its regis-
tered oce in Basel, comply with the requirements of
the Swiss accounting legislation of the Swiss Code of
Obligations (SCO).
Novartis AG is presenting consolidated financial
statements according to IFRS. Therefore, Novartis AG
has applied the exemption included in article 961d, para-
graph 1 SCO, and has not prepared additional disclo-
sures, a separate cash flow statement and a manage
-
ment report for SCO purposes.
2. Accounting policies
Financial income and expenses
Current assets and current liabilities denominated in
foreign currencies are converted at year-end exchange
rates. Realized exchange gains and losses, and all
unreali zed exchange losses arising from these as well
as those from business transactions, are recorded net
as financial income or financial expenses.
Derivative financial instruments
Derivative financial instruments are used for hedging pur-
poses. These instruments are valued at fair value. When
dierent accounting policies apply for the hedged item
and the derivative financial instrument, hedge accounting
is applied through measuring the hedged item together
with the derivative financial instrument.
Financial assets
Financial assets are valued at acquisition cost less
adjustments for foreign currency losses and any other
impairment of value.
Investments
Investments are initially recognized at cost. Investments
in Novartis Group subsidiaries are assessed annually
and, in case of an impairment, adjusted to their recover-
able amount within their category.
Goodwill
Goodwill is capitalized and amortized over a period of
20 years. Goodwill is reviewed for impairment on an
annual basis. If necessary, an impairment loss is recog-
nized.
Bonds
Bonds are valued at nominal value. Any bond premium
is accrued over the duration of the bond so that at
maturity, the balance sheet amount will equal the amount
that is due to be paid.
Provisions
Provisions are made to cover general business risks of
the Group.
Notes to the financial statements ofNovartisAG
A-4
3. Goodwill
(CHF millions)
Goodwill
Gross cost
Accumulated amortization
January –
–
Amortization charges –
–
December –
–
Net book value at December
1
There was no change during 2022 and 2021.
4. Financial income and expenses
2022 2021
(CHF millions) Income
Expenses
Income
Expenses
Interest 525
– 160
428
– 159
Foreign exchange 31
14
Others
– 1
Total 556
– 160
442
– 160
5. Investments
The principal direct and indirect subsidiaries and other holdings of Novartis AG are shown in Note 31 to the Group’s
consolidated financial statements.
6. Interest-bearing current receivables and liabilities
and financial assets
Interest-bearing current receivables and liabilities with
Group subsidiaries contain intragroup arrangements
under which the company grants or receives credits that
are available on demand.
Financial assets with Group subsidiaries include
financing arrangements and loans to direct or indirect
subsidiaries of Novartis AG.

Notes to the financial statements ofNovartisAG
A-5
7. Bonds
Straight bonds
Nominal
Issuance
Maturity
CHF
CHF
Coupon Currency amount
year
year
Issuer Issue price
millions
millions
CHF
Novartis AG, Basel, Switzerland
CHF
Novartis AG, Basel, Switzerland
CHF
Novartis AG, Basel, Switzerland
Total straight bonds
Breakdowns by maturity
(CHF millions)
After
Total
Comparison of balance sheet and fair value
(CHF millions) Balance sheet
Fair value
Balance sheet
Fair value
Straight bonds
Total
8. Share capital
2022 2021
Number
Share capital
Number
Share capital
of shares
CHF millions
of shares
CHF millions
January
Number of shares canceled/capital reduced during the period –
–
–
–
December
The Novartis AG share capital consists of registered
shares with a nominal value of CHF0.50 each.
The total share capital decreased from CHF1 217.2
million at December 31, 2021, to CHF1 201.9 million at
December 31, 2022, due to a share capital reduction as
a result of the cancellation of 30.7 million repurchased
shares with a nominal value of CHF 15.3 million. The
cancellation was approved at the Annual General Meeting
on March 4, 2022, and became eective on May 11, 2022.
During 2021, the total share capital decreased from
CHF1 233.5 million at December 31, 2020, to CHF1 217.2
million at December 31, 2021, due to a share capital
reduction as a result of the cancellation of 32.6 million
repurchased shares with a nominal value of CHF16.3
million. The cancellation was approved at the Annual
General Meeting on March 2, 2021, and became eec-
tive on July 8, 2021.

Notes to the financial statements ofNovartisAG
A-6
9. Treasury shares
2022 2021
Legal reserve for
Legal reserve for
treasury shares
treasury shares
Number
held by subsidiaries
Number
held by subsidiaries
of shares
CHF millions
of shares
CHF millions
Treasury shares held by subsidiaries
January
Number of shares purchased/sold; reserves transferred –
–
–
–
December
1
Excluding foundations
2022 2021
Deduction from equity
Deduction from equity
for treasury shares
for treasury shares
Number
held by Novartis AG
Number
held by Novartis AG
of shares
CHF millions
of shares
CHF millions
Treasury shares held by Novartis AG
January
Number of shares purchased/canceled; reserves transferred
–
–
December
2022 2021
Total
Total
Number of
treasury shares
Number
treasury shares
shares
CHF millions
of shares
CHF millions
Total treasury shares
January
Total number of shares purchased/sold or canceled;
reserves transferred
–
–
December
1
Excluding foundations
Novartis AG has met the legal requirements for legal
reserves under articles 659 et. seq. and 663b.10 SCO
for the treasury shares.
Treasury share purchases during 2022 totaled 127.7
million (2021: 32.2 million), with an average purchase
price of CHF82 (2021: CHF82). No treasury share sales
were executed during 2022 and 2021. Share-based
compensation transactions totaled 9.7 million shares
(2021: 9.9 million shares).
The number of treasury shares held by the Company
and its subsidiaries meet the definitions and require-
ments of article 659b SCO. As at December 31, 2022,
treasury shares held by Novartis AG and its fully-owned
subsidiaries totaled 185 080 017. It should be noted that
within the Novartis Group’s IFRS consolidated financial
statements, some Novartis entities are included in the
consolidation scope. These entities are mainly founda-
tions, which as at December 31, 2022 did not qualify as
subsidiaries in the sense of article 659b SCO.
With eective date of January 1, 2023, article 659b
SCO was amended to change the definition of subsid-
iaries to include foundations of the Company. This
change will have the impact as of January 1, 2023 to
increase the reported number of treasury shares held
by subsidiaries to reflect the Company foundations’
Novartis AG shares held (January 1, 2023: 96 969 226).
As of the entry into force of the revised Swiss corpo-
rate law on January 1, 2023, Novartis ordinary shares
held by Swiss foundations controlled by Novartis will no
longer carry the right to vote and therefore will be
included as treasury shares for determining compliance
with the legal requirements for legal reserves under arti-
cles 659 et. seq. and 663b.10 SCO for the treasury
shares.
For further information related to the amendment to
SCO article 659b, see Note 10.

Notes to the financial statements ofNovartisAG
A-7
10. Free reserves
(CHF millions)
January
Reduction due to cancellation of treasury shares (CHF million / CHF million of repurchased shares
less their nominal value of CHF million / CHF million) –
–
Transfer from legal reserve for treasury shares
December
1
Transfer from legal reserve for treasury shares (including expired dividends)
With eective date of January 1, 2023, article 659b
SCOwas amended to change the definition of subsid-
iaries to include foundations of the Company. This
change will have the impact as of January 1, 2023 to
increase the amount of legal reserves by the cost basis
of the treasury shares held by subsidiaries in the amount
of CHF2246 million, for the 96969226 Novartis AG
shares held by Company foundations, (from CHF 450
million to CHF 2 696 million), with a corresponding
decrease in free reserves of CHF 667 million and retained
earnings of CHF 1579 million.
11. Contingent liabilities
(CHF millions) Dec ,
Dec ,
Guarantees in favor of subsidiaries to cover capital and interest of bonds, credit facilities and commercial paper
programs – total maximum amount CHF million (: CHF million)
Other guarantees in favor of subsidiaries, associated companies and others –
total maximum amount CHF million (: CHF million)
Total contingent liabilities
Novartis AG is part of the Swiss Novartis value-added
tax (VAT) group and is therefore jointly liable for existing
and future VAT claims from the Swiss Federal Tax
Administration.
In December 2021, Novartis AG entered into an irre-
vocable, non-discretionary arrangement with a bank to
repurchase Novartis AG shares on the second trading
line under its up-to USD 15.0 billion share buyback. The
arrangement was updated in July 2022. Novartis AG is
able to cancel this arrangement but would be subject to
a 90-day waiting period under certain conditions. There
was no requirement to record a contingent liability under
this arrangement.

Notes to the financial statements ofNovartisAG
A-8
12. Registration, voting restrictions
andmajorshareholders
The Company’s Articles of Incorporation state that no
person or entity shall be registered with the right to vote
for more than 2% of the share capital, as set forth in the
commercial register. In particular cases, the Board of
Directors may allow exemptions from the limitation for
registration in the Novartis Share Register.
According to the Novartis Share Register, sharehold-
ers who owned 2% or more of the Company’s capital at
December31, 2022, and were entitled to voting rights on
all of their shares, excluding treasury shares held by
Novartis AG or its fully owned subsidiaries, were as fol-
lows:
% holding of
% holding of
share capital
share capital
Dec 31, 2022
Dec 31, 2021
Shareholders registered for
their own account:
Emasan AG, Basel 3.7
3.7
UBS Fund Management
(Switzerland) AG, Basel 2.3
2.3
Credit Suisse Funds AG, Zurich 2.1
2.1
Furthermore, there were the following other significant
share holders:
% holding of
% holding of
share capital
share capital
Dec 31, 2022
Dec 31, 2021
Shareholders registered as nominees:
Chase Nominees Ltd., London 8.4
8.8
Nortrust Nominees Ltd., London 3.8
4.2
The Bank of New York Mellon, New York 2.9
3.0
Through The Bank of New York Mellon, Everett 1.6
1.6
Through The Bank of New York Mellon, New York 0.9
1.1
Through The Bank of New York Mellon,
SA/NV, Brussels 0.4
0.3
Shareholder acting as American Depositary Share (ADS) depositary:
JPMorgan Chase Bank, N.A., New York 9.4
11.1
The following shareholder was disclosed through a noti-
fication filed with Novartis AG, but was not registered as
of December 31, 2022, in the Novartis Share Register:
• Norges Bank (Central Bank of Norway), Oslo, which
held 2.3% (2021: 2.1%)
The following shareholder was disclosed through a noti-
fication filed with Novartis AG and the SIX Swiss
Exchange, but was registered with less than 2% of the
share capital as of December 31, 2022, in the Novartis
Share Register:
• BlackRock, Inc., New York, which held between 5% and
10%

Notes to the financial statements ofNovartisAG
A-9
13. Equity instrument disclosures for the Board of
Directors and Executive Committee members
Share ownership requirements for Board members
The Chairman is required to own a minimum of 30 000
Novartis shares, and other members of the Board of
Directors are required to own at least 5 000 Novartis
shares within five years after joining the Board of Direc-
tors, to ensure their interests are aligned with those of
shareholders.
Board members are prohibited from hedging or
pledging their ownership positions in Novartis shares
that are part of their guideline share ownership require-
ment, and are required to hold these shares for 12 months
after retiring from the Board of Directors. As at Decem-
ber 31, 2022, all current and former members of the
Board of Directors who were required to meet the mini-
mum share ownership requirements did so.
Shares, ADRs and share options owned by Board
members
As at December 31, 2022, no member of the Board of
Directors, either individually or together with “persons
closely linked”
1
to them, owned 1% or more of the out-
standing shares (or ADRs) of Novartis. As at the same
date, no member of the Board of Directors held any share
options to purchase Novartis shares.
The total number of vested Novartis shares and ADRs
owned by members of the Board of Directors and
“ persons closely linked”
1
to them as at December 31,
2022, and as at December 31, 2021, is shown in the table
below.
Shares and ADRs owned by Board members
1
Number of shares
1,2
At
At
December 31,
December 31,
2022
2021
Joerg Reinhardt
3
632 730
609 156
Simon Moroney 4 102
2 240
Patrice Bula 8 802
6 543
Nancy C. Andrews 8 931
7 257
Ton Buechner 20 461
17 856
Elizabeth Doherty 12 836
10 743
Bridgette Heller 4 296
2 655
Daniel Hochstrasser 804
0
Frans van Houten 14 442
10 813
Ana de Pro Gonzalo 823
0
Andreas von Planta 168 717
166 390
Charles L. Sawyers 15 888
14 214
William T. Winters 27 659
24 436
Sub-Total 920 491
872 303
Board members who stepped down at the 2022 AGM
Enrico Vanni 32 078
30 965
Ann Fudge 12 751
13 222
Sub-Total 44 829
44 187
Total 965 320
916 490
na – not applicable
1
Includes holdings of “persons closely linked” to Board members (see the “persons
closely linked” definition).
2
Each share provides entitlement to one vote.
3
The 2021 Annual Report included an underestimated number of owned shares for
Joerg Reinhardt. It should stipulate 609 156 shares owned compared to 418 706 as
reported.
Share ownership requirements for Executive
Committee members
Executive Committee members are required to own at
least a minimum multiple of their annual base salary in
Novartis shares or RSUs within five years of hire or pro-
motion, as set out in the table below. In addition, the CEO
and CFO are required to hold the equity vesting under
the LTPP plan (granted since 2021) for a minimum of two
years after the vesting date. In the event of a substantial
rise or drop in the share price, the Board of Directors
may, at its discretion, amend that time period accord-
ingly.
Function Ownership level
CEO 5 x base compensation
Other Executive Committee members 3 x base compensation

Notes to the financial statements ofNovartisAG
A-10
The determination of equity amounts against the share
ownership requirements is defined to include vested and
unvested Novartis shares or American Depositary
Receipts (ADRs), and RSUs acquired under the Compa-
ny’s compensation plans. However, unvested PSUs are
excluded. The determination also includes other shares
and vested options of Novartis shares or ADRs that are
owned directly or indirectly by “persons closely linked”
to an Executive Committee member. The Compensation
Committee reviews compliance with the share owner-
ship guideline on an annual basis.
As at December 31, 2022, all members who have
served at least five years on the Executive Committee
have met or exceeded their personal Novartis share own-
ership requirements.
Shares, ADRs, equity rights and share options
owned by Executive Committee members
As at December 31, 2022, no member of the Executive
Committee, either individually or together with “persons
closely linked”
1
to them, owned 1% or more of the out-
standing shares (or ADRs) of Novartis. As at the same
date, no member of the Executive Committee held any
share options to purchase Novartis shares.
The following table shows the total number of shares,
ADRs and other equity rights owned by Executive
Committee members and “persons closely linked”
1
to
them as at December 31, 2022, and as at December 31,
2021.
1
“Persons closely linked” are (i) their spouse, (ii) their children below age 18, (iii) any
legal entities that they own or otherwise control, and (iv) any legal or natural person
who is acting as their fiduciary.
Shares, ADRs and other equity rights owned by Executive Committee members
1
Unvested
Unvested
Vested
shares
Total as at
Vested
shares
Total as at
shares
and other
December ,
shares
and other
December 31,
and ADRs
equity rights
and ADRs
equity rights
2021
Vasant Narasimhan
Shreeram Aradhye (from May , )
Victor Bulto (from May , )
Aharon Gal (from July , )
Karen Hale
Harry Kirsch
Robert Kowalski
Steen Lang
Fiona Marshall (from November , )
Klaus Moosmayer
Marie-France Tschudin
Subtotal
Executive Committee members who stepped down during 2022
James Bradner (until October , )
Richard Saynor (until October , )
Susanne Schaert (until April , )
John Tsai (until May , )
Robert Weltevreden (until April , )
Subtotal
Total
1
Includes holdings of “persons closely linked” to Executive Committee members (see “—persons closely linked” definition).
2
Includes restricted shares, RSUs and target number of PSUs. Target number of PSUs are disclosed pro-rata to December 31, unless the award qualified for full vesting under the
relevant plan rules. Awards under all other incentive plans are disclosed in full.
3
Excludes members who stepped down during the year.
4
The 2021 Annual Report included an underestimated number of owned shares for Susanne Schaert and Jon Tsai. It should respectively stipulate 120 003 and 25 768 shares
owned compared to 116 173 and 23 382 as reported.

Appropriation of available earnings and reserves ofNovartis AG
A-11
Appropriation of available earnings and
reserves ofNovartis AG
Appropriation of available earnings of Novartis AG as
per balance sheet and declaration of dividend
(CHF)
Available unappropriated earnings
Balance brought forward before capital reduction
Reduction due to cancellation of treasury shares
–
–
Net income of the year
Total available earnings at the end of the year
Transfer to legal reserves for treasury shares
–
Total available earnings at the disposal of the Annual General Meeting
Appropriation proposed by the Board of Directors
Payment of a gross dividend (before taxes and duties) of CHF (: CHF ) on
(: ) dividend-bearing shares
with a nominal value of CHF each –
–
Total available earnings after appropriation
Dividend waived for additional treasury shares held by the Company
Balance to be carried forward
1
Based on the Annual General Meeting resolution of March 4, 2022 and March 2, 2021
2
With eective date of January 1, 2023, article 659b SCO was amended to change the definition of subsidiaries to include foundations of the Company. This amendment requires an
additional allocation of legal reserve for treasury shares held by foundations as of January 1, 2023, resulting in a reduction in available earnings at the disposal of the Annual
General Meeting
3
No dividend will be declared on treasury shares held by Novartis AG or its fully owned subsidiaries
If this proposal is approved, the dividend will be paid as from March 13, 2023. The last trading day with entitlement
to receive the dividend is March 8, 2023. As from March 9, 2023, the shares will be traded ex-dividend.

Statutory Auditor’s Report
A-12
Statutory Auditor’s Report
to the General Meeting of NovartisAG
Basel
Report on the Audit of the Financial
Statements
Opinion
We have audited the financial statements of Novartis AG
(the Company), which comprise the balance sheet as at
December 31, 2022, and the income statement for the
year then ended, and notes to the financial statements,
including a summary of significant accounting policies.
In our opinion, the financial statements (pages A-1 to
A-11) comply with Swiss law and the Company’s articles
of incorporation.
Basis for Opinion
We conducted our audit in accordance with Swiss law
and Swiss Standards on Auditing (SACH). Our respon-
sibilities under those provisions and standards are fur-
ther described in the “Auditor’s Responsibilities for the
Audit of the Financial Statements” section of our report.
We are independent of the Company in accordance with
the provisions of Swiss law, together with the require-
ments of the Swiss audit profession and we have fulfilled
our other ethical responsibilities in accordance with
these requirements.
We believe that the audit evidence we have obtained
is sucient and appropriate to provide a basis for our
opinion.
Key Audit Matters
Key audit matters are those matters that, in our profes-
sional judgment, were of most significance in our audit
of the financial statements of the current period. We have
determined that there are no key audit matters to com-
municate in our report.
Other Matter
The financial statements of Novartis AG for the year
ended December 31, 2021 were audited by another audi-
tor who expressed an unmodified opinion on those state-
ments on February 1, 2022.
Other Information in the Annual
Report
The Board of Directors is responsible for the other infor-
mation in the Annual Report. The other information com-
prises the information included in the annual report, but
does not include the consolidated financial statements,
the stand-alone financial statements of the Company,
the compensation report and our auditor’s reports
thereon.
Our opinion on the financial statements does not
cover the other information in the Annual Report and we
do not express any form of assurance conclusion
thereon.
In connection with our audit of the financial state-
ments, our responsibility is to read the other information
in the Annual Report and, in doing so, consider whether
the other information is materially inconsistent with the
financial statements or our knowledge obtained in the
audit or otherwise appears to be materially misstated.
If, based on the work we have performed, we con-
clude that there is a material misstatement of this other
information, we are required to report that fact. We have
nothing to report in this regard.
Board of Directors’ Responsibilities
for the Financial Statements
The Board of Directors is responsible for the prepara-
tion of the financial statements in accordance with the
provisions of Swiss law and the Company’s articles of
incorporation, and for such internal control as the Board
of Directors determines is necessary to enable the
preparation of financial statements that are free from
material misstatement, whether due to fraud or error.
In preparing the financial statements, the Board of
Directors is responsible for assessing the Company’s
ability to continue as a going concern, disclosing, as
applicable, matters related to going concern and using
the going concern basis of accounting unless the Board
of Directors either intends to liquidate the Company or
to cease operations, or has no realistic alternative but to
do so.
Auditor’s Responsibilities for the
Audit of the Financial Statements
Our objectives are to obtain reasonable assurance about
whether the financial statements as a whole are free from
material misstatement, whether due to fraud or error, and
to issue an auditor’s report that includes our opinion.
Reasonable assurance is a high level of assurance, but
is not a guarantee that an audit conducted in accordance
with Swiss law and SACH will always detect a material
misstatement when it exists. Misstatements can arise
from fraud or error and are considered material if, indi-
vidually or in the aggregate, they could reasonably be
expected to influence the economic decisions of users
taken on the basis of these financial statements.

Statutory Auditor’s Report
A-13
As part of an audit in accordance with Swiss law and
SACH, we exercise professional judgment and maintain
professional skepticism throughout the audit. We also:
• Identify and assess the risks of material misstatement
of the financial statements, whether due to fraud or
error, design and perform audit procedures responsive
to those risks, and obtain audit evidence that is su-
cient and appropriate to provide a basis for our opin-
ion. The risk of not detecting a material misstatement
resulting from fraud is higher than for one resulting
from error, as fraud may involve collusion, forgery,
intentional omissions, misrepresentations, or the over-
ride of internal control.
• Obtain an understanding of internal control relevant to
the audit in order to design audit procedures that are
appropriate in the circumstances, but not for the pur-
pose of expressing an opinion on the eectiveness of
the Company’s internal control.
• Evaluate the appropriateness of accounting policies
used and the reasonableness of accounting estimates
and related disclosures made.
• Conclude on the appropriateness of the Board of
Directors’ use of the going concern basis of account-
ing and, based on the audit evidence obtained, whether
a material uncertainty exists related to events or con-
ditions that may cast significant doubt on the Compa-
ny’s ability to continue as a going concern. If we con-
clude that a material uncertainty exists, we are required
to draw attention in our auditor’s report to the related
disclosures in the financial statements or, if such dis-
closures are inadequate, to modify our opinion. Our
conclusions are based on the audit evidence obtained
up to the date of our auditor’s report. However, future
events or conditions may cause the Company to cease
to continue as a going concern.
We communicate with the Board of Directors, primarily
through the Audit and Compliance Committee regard-
ing, among other matters, the planned scope and timing
of the audit and significant audit findings, including any
significant deficiencies in internal control that we iden-
tify during our audit.
We also provide the Board of Directors or its relevant
committee with a statement that we have complied with
relevant ethical requirements regarding independence,
and communicate with them all relationships and other
matters that may reasonably be thought to bear on our
independence, and where applicable, actions taken to
eliminate threats or safeguards applied.
From the matters communicated with the Board of
Directors or its relevant committee, we determine those
matters that were of most significance in the audit of the
financial statements of the current period and are there-
fore the key audit matters. We describe these matters in
our auditor’s report, unless law or regulation precludes
public disclosure about the matter or when, in extremely
rare circumstances, we determine that a matter should
not be communicated in our report because the adverse
consequences of doing so would reasonably be expected
to outweigh the public interest benefits of such commu-
nication.
Report on Other Legal and
Regulatory Requirements
In accordance with article 728a para. 1 item 3 CO and
PSCH 890, we confirm that an internal control system
exists, which has been designed for the preparation of
financial statements according to the instructions of the
Board of Directors.
We further confirm that the proposed appropriation
of available earnings complies with Swiss law and the
Company’s articles of incorporation. We recommend that
the financial statements submitted to you be approved.
KPMG AG
Richard Broadbelt Norman Dittes
Licensed Audit expert Licensed Audit Expert
Auditor in Charge
Basel, January 31, 2023

